

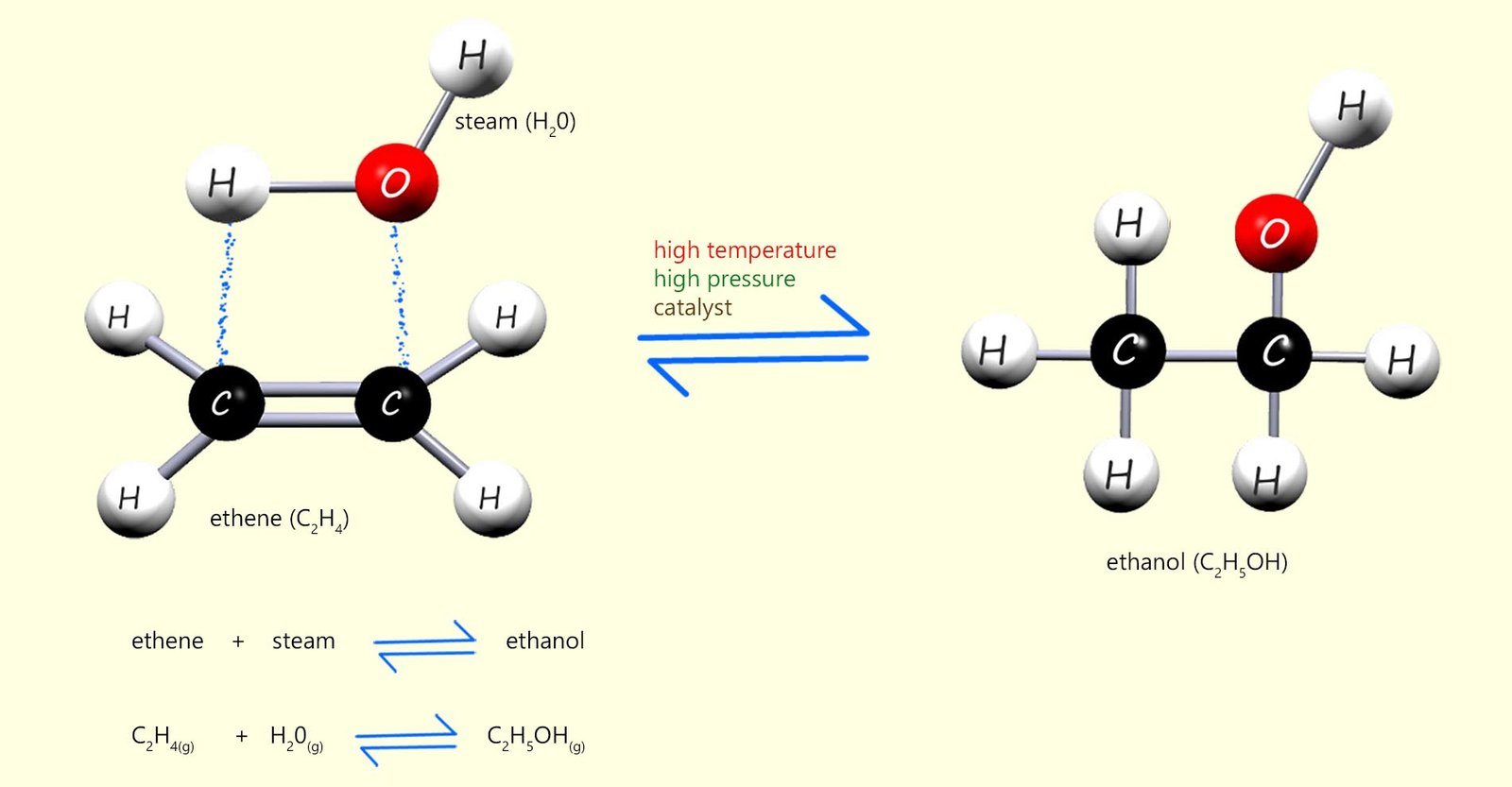
There are numerous methods of preparing alcohols from alkenes and we will investigate some of these on this page. One method which is often used in industry is called direct hydration. This involves adding a molecule of water across the carbon-carbon double bond (C=C) in an alkene to produce an alcohol, for example the image below shows how a molecule of water can add across the carbon carbon double bond in a molecule of ethene to form the alcohol ethanol; this method requires:
The image below outlines how a molecule of water can add directly across the C=C bond in ethene to form ethanol:
💧 An oxonium ion forms when a molecule containing oxygen accepts a proton (H⁺); H₃O⁺ is the oxonium ion formed from water and is called the hydronium ion.
The mechanism for this reaction is shown below. The mechanism is similar to the other electrophilic addition reactions to alkenes and proceeds via three steps:
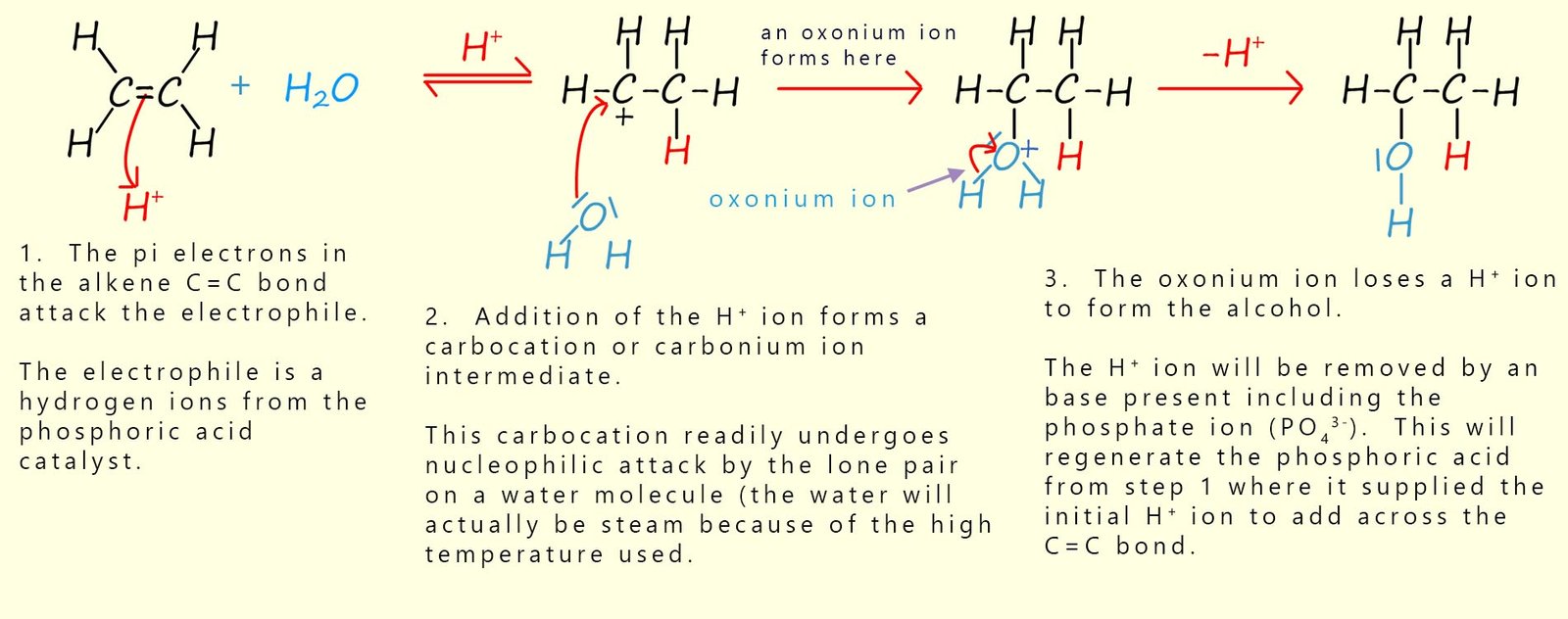
Complete the activity below by putting the mechanism steps for the direct hydration mechanism in the correct order.
Put the steps for the direct hydration of an alkene into the correct order by clicking them one at a time.
Alcohols can also be produced from alkenes using concentrated sulfuric acid, however this reaction is not carried out in a single step as is the case with direct hydration of alkenes. In practice, the reaction is performed in two stages.
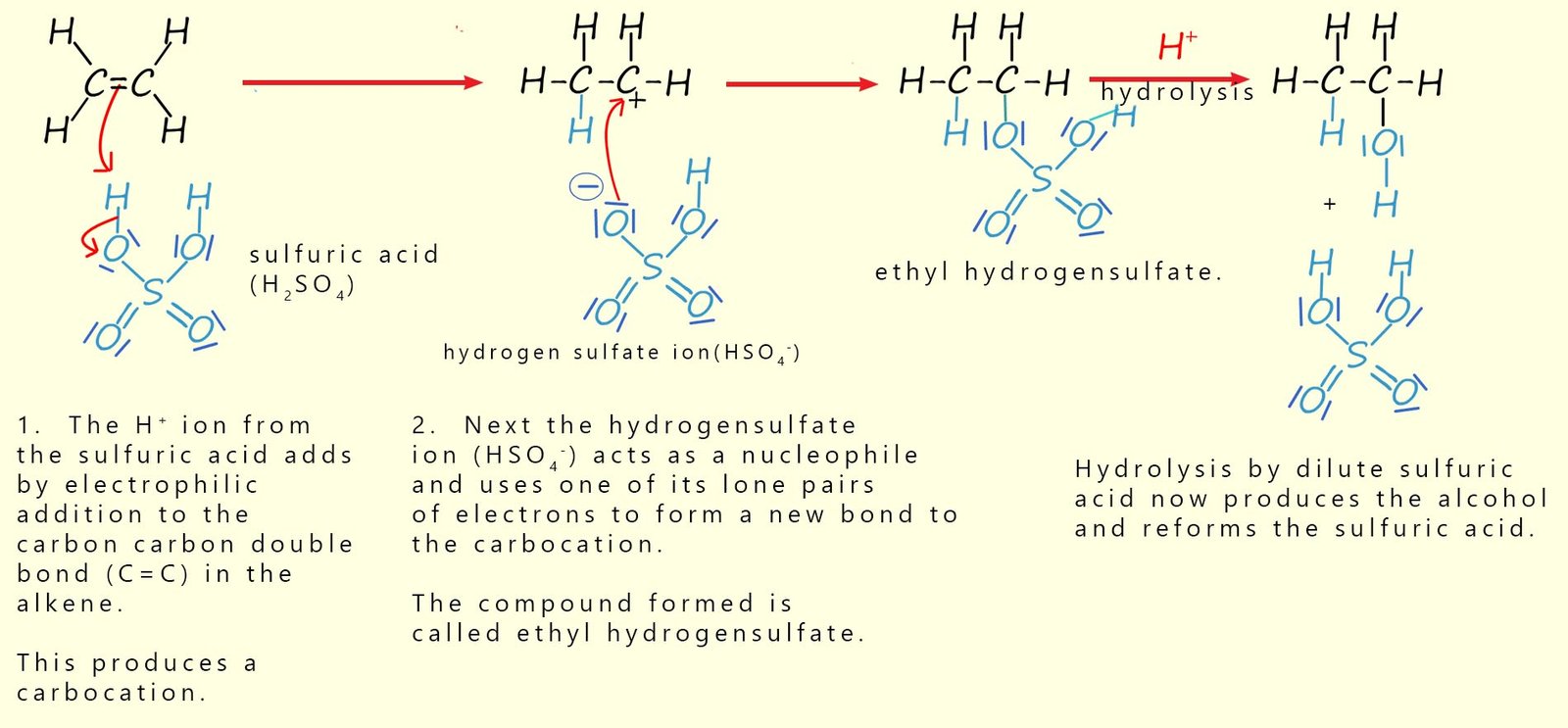
Step 1: Cold, concentrated sulfuric acid is added to the alkene. The alkene undergoes an electrophilic addition reaction in which the pi bond in the alkene molecule is attacked by a hydrogen ion (H+) from the sulfuric acid. This produces a carbocation intermediate, which then reacts with the hydrogensulfate ion (HSO4−) to form an alkyl hydrogensulfate.
Step 2: The alkyl hydrogensulfate is then warmed with water or steam. This causes hydrolysis, that is the breaking of the bond to the hydrogensulfate group and this forms the alcohol. Sulfuric acid is regenerated in this step, so it acts as a catalyst overall.
The mechanism for the preparation of ethanol from the alkene ethene using concentrated sulfuric acid as a catalyst is outlined below:
The following section of work is NOT examined in the AQA, OCR or Edexcel A-level chemistry specifications, so you may well ask why it is included. The answer is simple: questions based on this chemistry have appeared in A-level chemistry past papers. So if you have a spare 10 minutes, why not increase your already vast knowledge of chemistry — it’s actually interesting chemistry!
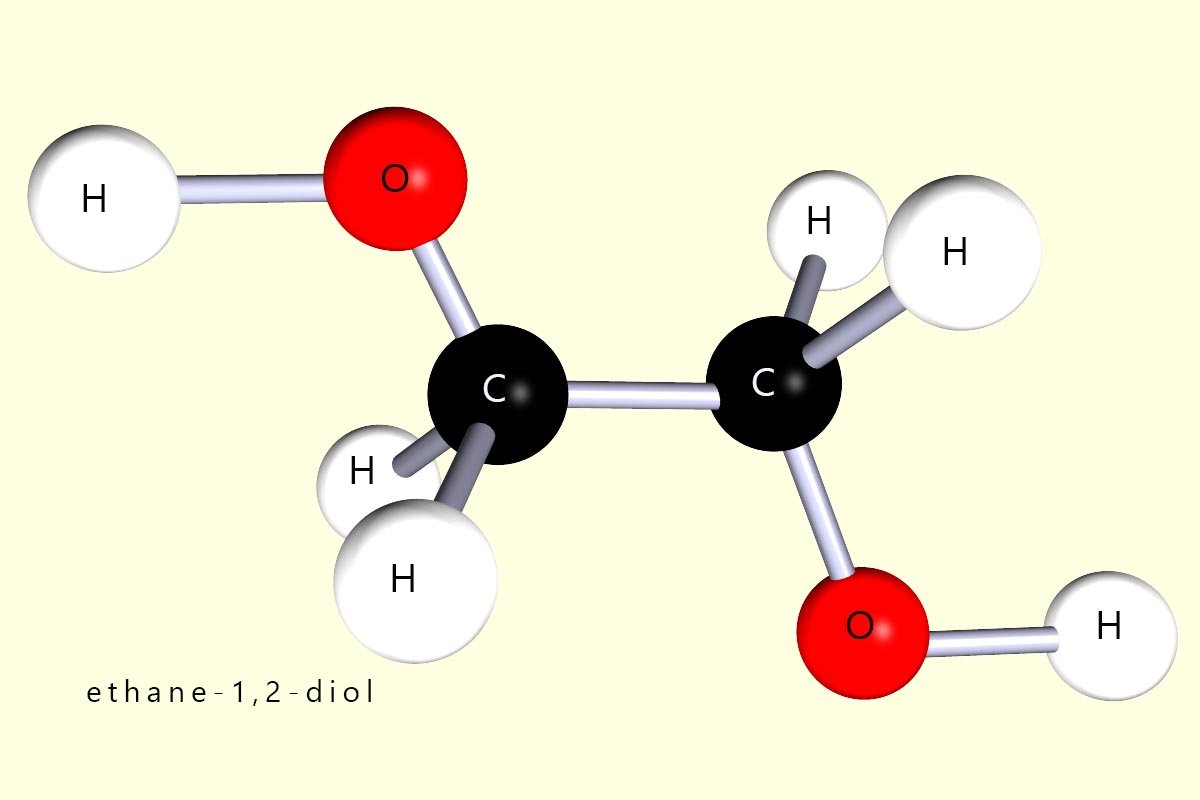
The diols are a family of alcohols which contain two hydroxyl functional groups (–OH) per molecule. Perhaps the most widely used diol is ethane-1,2-diol (CH2OHCH2OH), which is used as antifreeze and as a coolant in car engines. A model of this molecule is shown below:
Alkenes can be oxidised to form 1,2-diols using oxidising agents such as cold, dilute solutions of potassium permanganate. The permanganate ion (MnO4-) is a powerful oxidising agent simply because the central manganese atom has an oxidation state of +7. Potassium permanganate can be used in neutral or alkaline conditions to oxidise an alkene to form a diol.

In alkaline solution, the permanganate ion (MnO4-) can be reduced to form the green manganate ion MnO42-, especially in strongly alkaline conditions:
If excess alkene is present, the manganate ion can be further reduced as the alkene continues to act as a reducing agent. This leads to the formation of brown manganese dioxide (MnO2), which appears as a brown precipitate. The ethene is still oxidised to form the diol ethane-1,2-diol.
The permanganate ion adds to one side of the carbon–carbon double bond (C=C) in an alkene; this is called syn addition. We can show this as:
However, the complete equations for the oxidation of alkenes by potassium permanganate are not required at A-level, and we can simply show the equation for the oxidation reaction as:
Quick quiz to check your understanding of how alcohols are made from alkenes, simply click the button to open quick quiz.
Click Exam-safe or Exam trap. Then click Show explanation to see why.
1) “Acidified potassium permanganate oxidises an alkene to a 1,2-diol.”
Acidified permanganate is a much stronger oxidising agent and does not normally give diols. The diol product is associated with cold, dilute, neutral or alkaline permanganate.
2) “In electrophilic addition, the curly arrow starts at the pi bond in the C=C.”
The C=C pi bond is electron rich, so the curly arrow should start at the C=C and point towards the electrophile. Starting at the electrophile is a common exam error.
3) “Sulfuric acid is used up when making an alcohol from an alkene.”
In the two-step route, sulfuric acid is regenerated during hydrolysis, so it acts as a catalyst overall.
4) “Direct hydration adds H and OH across the C=C to form an alcohol.”
This is exam-safe as an overall description: water adds across the C=C to form an alcohol. Mechanistically, the alkene is protonated first, then water attacks, then a proton is lost.
Complete the activity below by simply matching the scenario to the correct method.
Click a scenario, then choose the correct method. Try to use the conditions as your clue.
| Method | Reagents / conditions | Main product | What to remember for exams |
|---|---|---|---|
| 🌫️ Direct hydration | steam, H3PO4 (catalyst), 570 K, 65 atm | Alcohol | Electrophilic addition via a carbocation then attack by water; catalyst is regenerated. |
| 🧪 Concentrated sulfuric acid route |
Step 1: conc H2SO4 (cold) adds across C=C Step 2: warm with water/steam (hydrolysis) |
Alcohol | Two-step method; forms an alkyl hydrogensulfate first. H2SO4 is regenerated overall. |
| 🟣 Oxidation with permanganate (enrichment) | cold, dilute KMnO4, neutral or alkaline | 1,2-diol | Purple permanganate is reduced; in alkaline conditions may see MnO2 (brown precipitate). Full equations not required. |
Before you move on, can you tick all of these?
✅ If you can tick all of these, you’re in a strong exam position.