Bond breaking or bond fission as it is also known can occur in two common ways; these are:
The image below explains in detail the difference between these two ways in which a covalent bond can be broken.
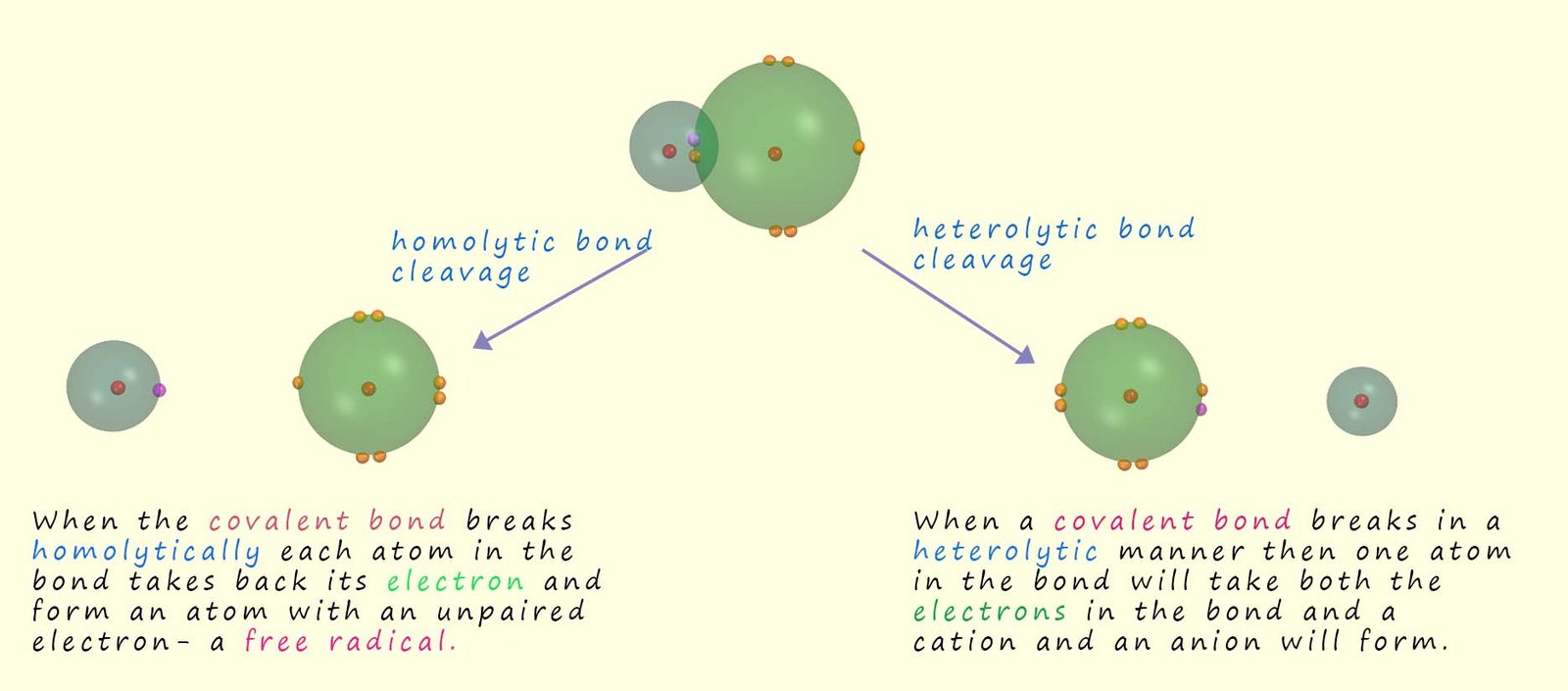
Heterolytic bond fission or cleavage usually occurs in covalent bonds when the electrons are unequally shared by the atoms involved in forming the covalent bond, this unequal sharing of the two electrons is due to differences in the electronegativity of the two atoms involved in the covalent bond. This means that when the covalent bond is broken one atom; the most electronegative atom in the bond takes the 2 electrons in the covalent bond. This will result in one atom gaining an electron and one atom losing an electron meaning that positively and negatively charged ions will be formed.
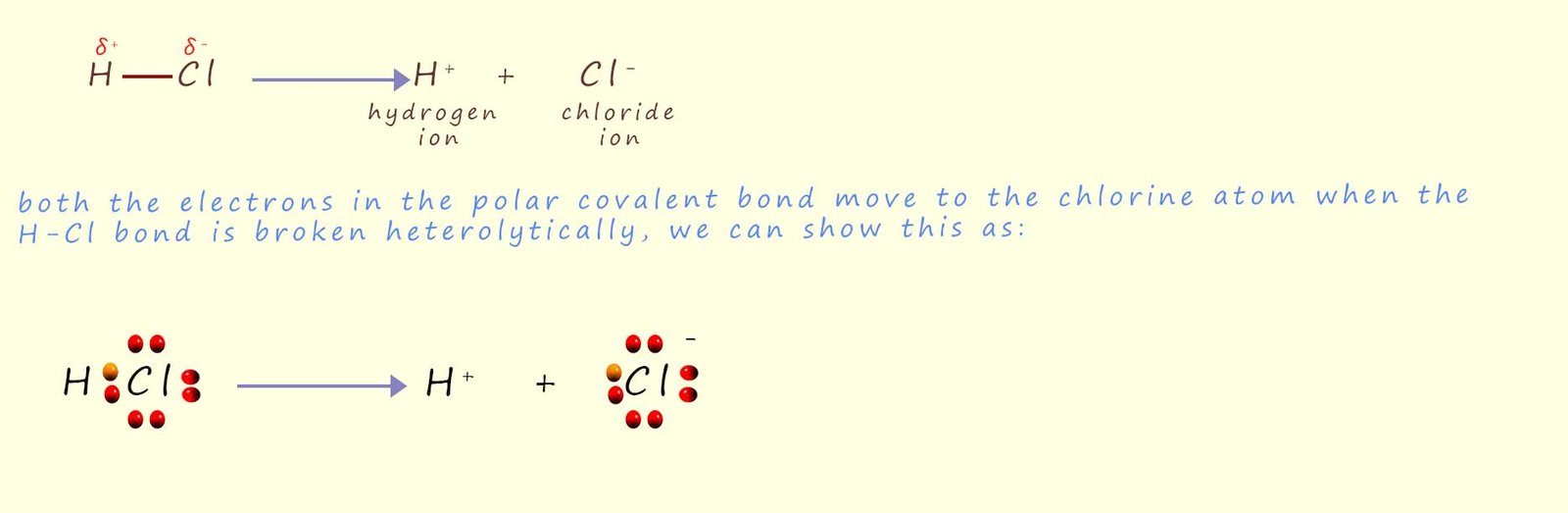
The bond between the two atoms in a hydrogen chloride molecule is a polar covalent one. Chlorine being more electronegative than hydrogen has a greater share of the two electrons in the polar covalent bond, so when this type of bond breaks the chlorine atom will take both electrons in the bond forming a negatively charged chloride anion and a positively charged hydrogen cation. This process which results in the formation of ions is called heterolytic bond fission and is outlined below.
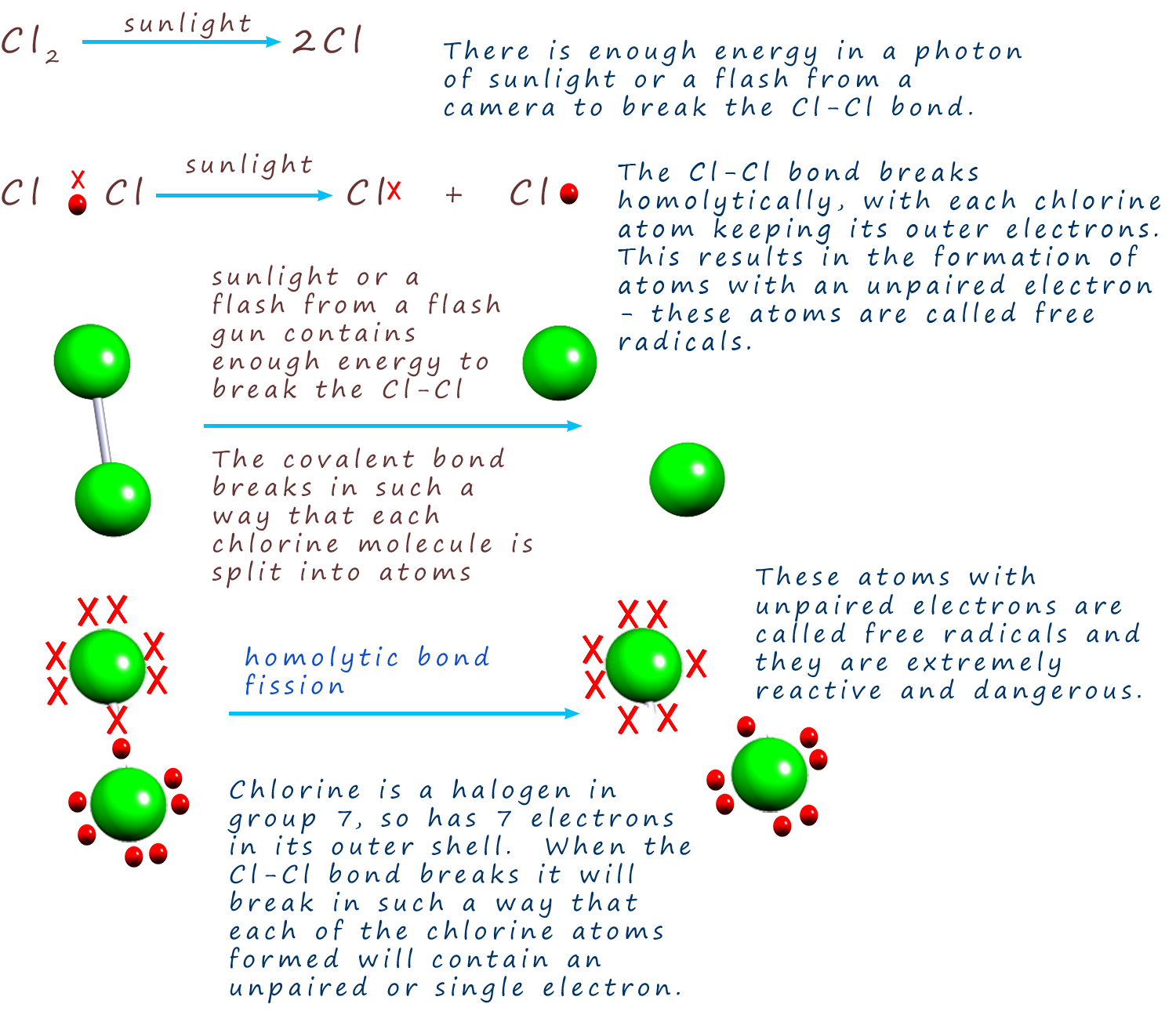 Homolytic bond fission tends to occurs in molecules
where there is covalent bonding present between atoms. This means that
the two electrons in the covalent bond
are equally shared. If this covalent bond is split then each atom
involved in forming the covalent bond will simply
take back its electron. This means that the "species" formed as a
result of this type of bond breaking will have an unpaired
single electron. These atoms with unpaired electrons are called
free radicals.
Homolytic bond fission tends to occurs in molecules
where there is covalent bonding present between atoms. This means that
the two electrons in the covalent bond
are equally shared. If this covalent bond is split then each atom
involved in forming the covalent bond will simply
take back its electron. This means that the "species" formed as a
result of this type of bond breaking will have an unpaired
single electron. These atoms with unpaired electrons are called
free radicals.
It is possible to break the covalent bonds in halogens such as
chlorine and bromine by simply exposing the molecules to
bright sunlight or shining
light from an artificial source such as that from a camera flash onto them. The diagram opposite shows how the bond in a chlorine
molecule can be broken in such a way as to produce two chlorine free radicals with
unpaired electrons with
unpaired electrons.
When the covalent bond breaks in such a way that each atom
involved in the bond simply takes back its electron we call this
homolytic bond fission or
homolytic bond cleavage.
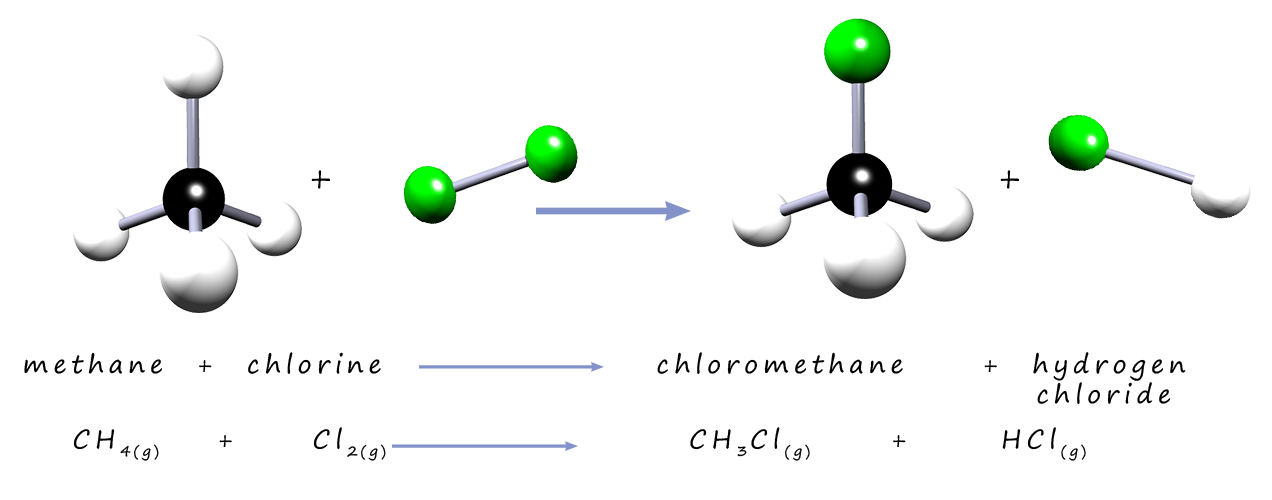
The alkanes are a homologous series of hydrocarbons that you met in gcse chemistry. They are generally unreactive molecules except when they are used as fuels in combustion reactions. They are unreactive with acids, alkalis, electrophiles and nucleophiles this is simply because they contain non-polar C-H bonds. However free radicals can react with alkane molecules to produce halogenalkanes or haloalkanes. Here one or more of the hydrogen atoms in the alkane molecule are replaced or substituted by a halogen atom in these explosive and violent reactions. This is outlined below:
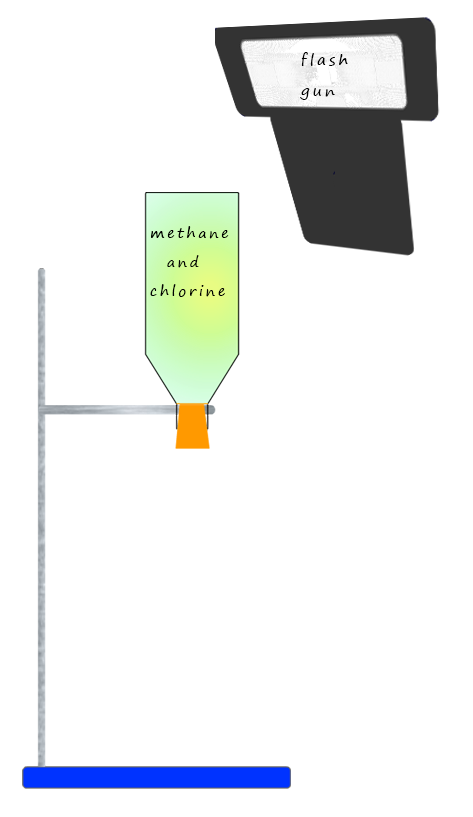
Methane (CH4) is the first member of the alkanes, it is the gas used in Bunsen burners in the lab and for heating and cooking in the home. If a mixture of methane and chlorine are placed in a plastic bottle in a dark room then no reaction will take place. However if a flash is produced from a flash gun then a bright orange flash is seen as a very rapid reaction takes place between the chlorine and the methane gases in the bottle. The products of this reaction are chloromethane (a halogenalkane) and hydrogen chloride gas, an equation for this free-radical substitution reaction is shown below. This reaction is a chain reaction and occurs through free radical intermediates which are produced by homolytic bond cleavage of a chlorine molecule.