

Higher and foundation tiers
 Your stomach contains hydrochloric acid.
Hydrochloric acid is a strong acid and even dilute solutions of it will have
a pH of one. If too much acid is produced in the stomach then you may feel a burning pain, this is called indigestion.
Antacids are tablets that are taken to relieve acid indigestion, but what is in these antacid or indigestion tablets to relieve the pain caused by
indigestion?
Your stomach contains hydrochloric acid.
Hydrochloric acid is a strong acid and even dilute solutions of it will have
a pH of one. If too much acid is produced in the stomach then you may feel a burning pain, this is called indigestion.
Antacids are tablets that are taken to relieve acid indigestion, but what is in these antacid or indigestion tablets to relieve the pain caused by
indigestion?
Well indigestion tablets contain a substance called a base.
Bases are substances which will neutralise an acid.
Examples of common
bases include substances such as metal oxides, metal carbonates and metal hydroxides. Most indigestion or
antacid tablets contain 500mg (0.5g) of calcium carbonate or chalk.
It might seem odd that to cure indigestion you swallow a
tablet containing chalk! But calcium carbonate or chalk is a harmless substance which in small amounts will not cause any
harm when eaten. Calcium carbonate is a base; it will neutralise any excess
hydrochloric or stomach acid and relieve the pain caused
by indigestion (For equations for these metal carbonate acid reactions click here).
Bases are substances which neutralise an acid. They react with acids to produce a salt and water. Common bases are solid metal oxides, metal carbonates and metal hydroxides. Some bases will dissolve in water; however most bases are insoluble and will not dissolve in water. If a base does dissolves in water it will form an alkaline solution, recall that an alkali is a solution which contains an excess of hydroxide ions (OH-) e.g. the table below lists some common bases you are likely to use in the science lab.
| Common bases | Soluble or Insoluble | Can it form an alkali? |
|---|---|---|
| copper oxide | insoluble | no |
| sodium oxide | soluble | yes |
| calcium carbonate | insoluble | no |
| sodium hydroxide | soluble | yes |
| potassium hydroxide | soluble | yes |

Notice that
all the soluble
bases in the table above will dissolve in water to form alkalis or alkaline solutions while the insoluble bases cannot form alkalis or alkaline solutions. Remember all alkalis are aqueous solutions, that is solutions formed by dissolving a substance in water.
Alkalis are solutions which contain an excess of hydroxide ions (OH-). It is the hydroxide ions present in which give alkalis their
characteristic properties: alkalis feel soapy or slippery to the touch although you should NOT touch most alkalis especially the strong alkalis with high pH values since they are
corrosive solutions. All alkalis have a pH above 7.
Weak alkalis such as ammonium hydroxide have a pH around 9 whereas strong alkalis such as lithium hydroxide, sodium hydroxide or potassium hydroxide have a higher pH; in the range 12-14. The colour chart for universal indicator is shown below; you can see that for alkalis the colours range from dark green for weak alkalis to a dark purple colour for strong alkalis. Recall that the higher the pH of an alkaline solution the higher the concentration of hydroxide ions (OH-(aq)) present in that solution.
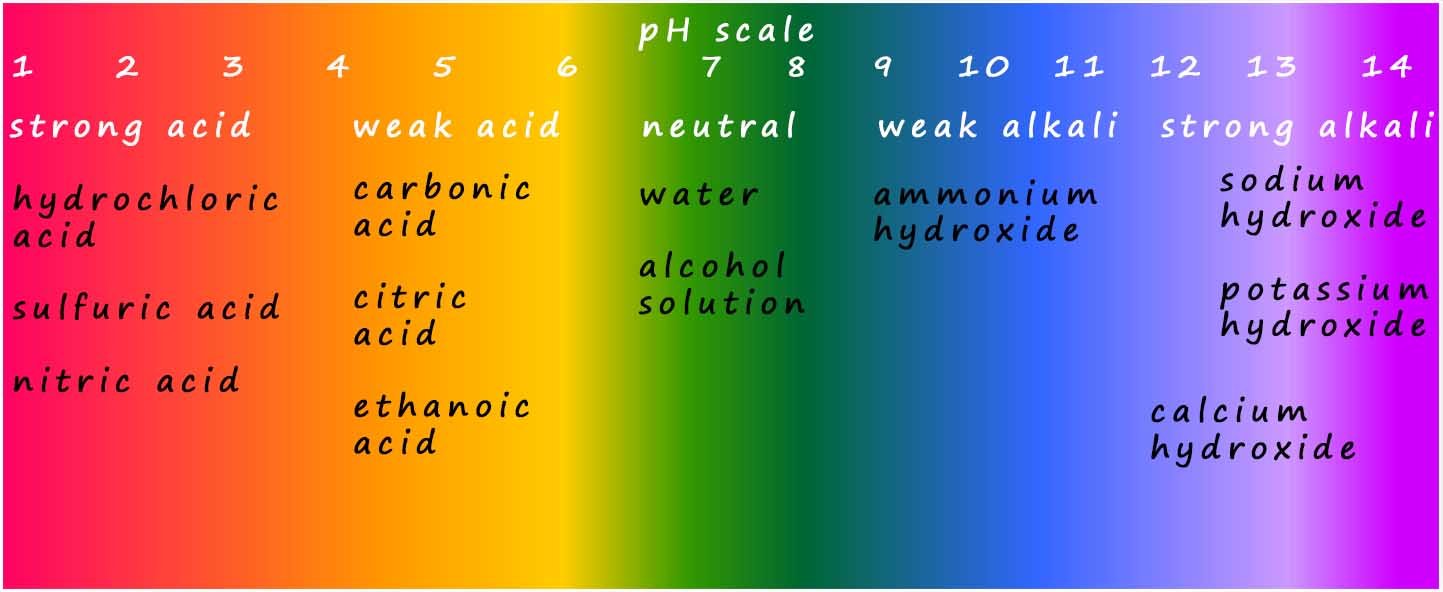
Alkalis are solutions formed when bases dissolve in water. One of the most common alkalis used in science labs is sodium hydroxide. This is formed when the base sodium oxide dissolves in water:

Ammonium hydroxide is another fairly common alkaline solution you are likely to come across in the chemistry lab. It is formed when the basic gas ammonia (NH3), which is highly soluble in water, dissolves to form the weak alkali ammonium hydroxide, as outlined in the equations below:
Test your understanding of the main ideas on bases and alkalis covered above by filling in the blanks to complete the paragraph below. The words to complete the blanks are shown in the yellow box and the drop down menus. Simply select the correct word from the drop-down menu.