

Higher and foundation tiers
Many of the physical properties of the alkanes depend on the size of the particular alkane molecule, these properties include: boiling and melting points, viscosity and flammability. Now viscosity is simply how runny or how easily a substance will flow and flammability which is how readily a substance catches fire. These properties are particularly important for hydrocarbons since their main use is as fuels. The other physical properties which we will look at are the melting and boiling points of the alkanes. The table below contains the melting and boiling points of the first 10 alkanes; can you spot any trends or patterns in the data?
| alkane | molecular formula | boiling point/0C | melting point point/0C | state at room temperature |
|---|---|---|---|---|
| methane | CH4 | -161 | -182 | gas |
| ethane | C2H6 | -88 | -183 | gas |
| ethane | C2H6 | -88 | -183 | gas |
| propane | C3H8 | -42 | -188 | gas |
| butane | C4H10 | -0.5 | -138 | gas |
| pentane | C5H12 | 36 | -130 | liquid |
| hexane | C6H14 | 69 | -95 | liquid |
| heptane | C7H16 | 98 | -90 | liquid |
| octane | C8H16 | 126 | -57 | liquid |
| nonane | C9H20 | 151 | -51 | liquid |
| decane | C10H22 | 174 | -30 | liquid |
The trends in the boiling point and melting points is fairly obvious; the larger the alkane molecule the higher the boiling and melting points. This trend is much as expected; the larger the molecule the larger it's molecular mass and the more intermolecular bonding there is between different molecules; so its boiling and melting point increases. This is outlined in the diagram below; where the two alkane molecules ethane and butane are shown. Butane being a larger molecule than ethane has more intermolecular bonding present between different butane molecules.
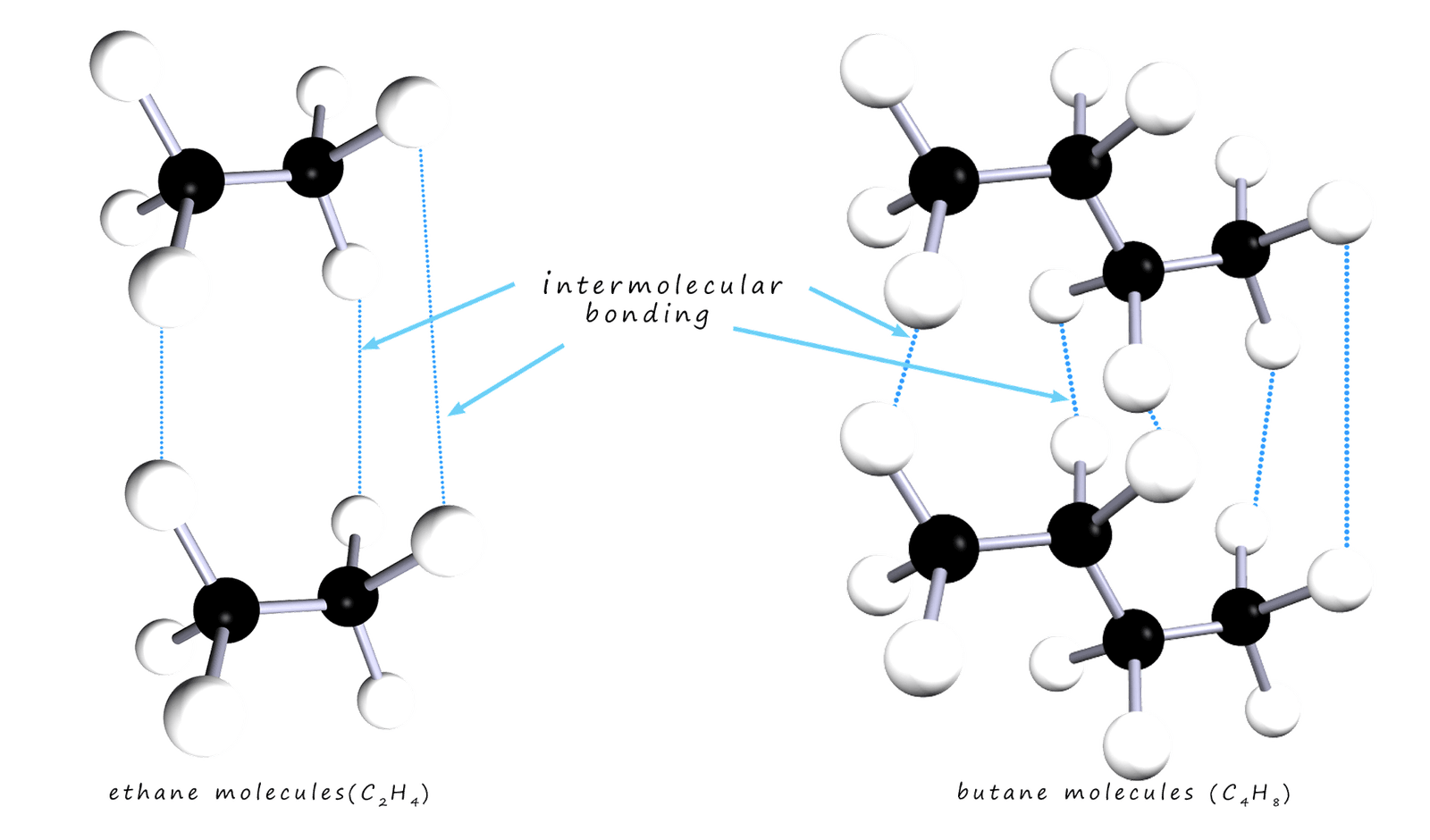
The general trends is similar with the melting points. The first 4 alkanes are all gases and pentane (C5H12) to hexadecane (C16H34) are all liquids at room temperature. The viscosity of the alkane liquids also changes with chain length; the shorter the chain length the more runny and the more easily it flows, that is it is less viscous. The longer the carbon chain the more viscous and thick is the liquid alkane.
A volatile substance is one that evaporates easily. The smaller the alkane molecule the lower will be its boiling point and being a small molecule will mean there is only a small amount of intermolecular bonding present. This means it will require only a small amount of energy to remove particles from the liquid state to the gaseous state. These small molecules will be volatile substances and will evaporate easily. This is outlined in the image below:
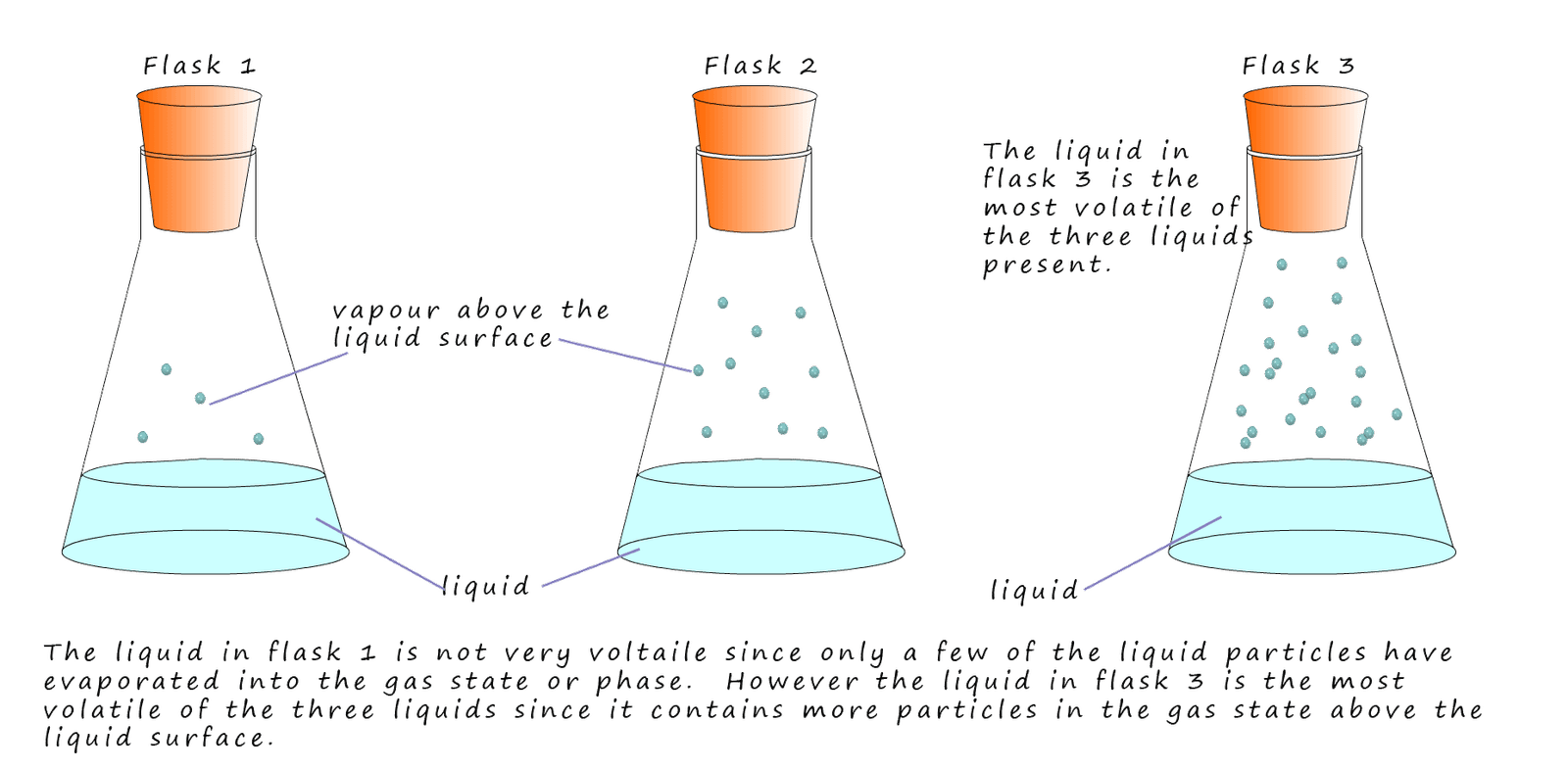
Alkanes which are liquids at room temperature and which contain small molecules will be runny; that is not particularly viscous, they will be volatile and evaporate easily. As the size of the alkane molecules increase then there will be more intermolecular bonding between the molecules and this along with their increase in mass will result in an increase in their viscosity and volatility.
 Alkanes are used mainly as fuels. Obviously if an alkane
is going to be used as a fuel it must
be flammable. But just how flammable are alkanes? Consider the following example:
Alkanes are used mainly as fuels. Obviously if an alkane
is going to be used as a fuel it must
be flammable. But just how flammable are alkanes? Consider the following example:
A liquid
is placed in an evaporating basin as shown
in the image opposite. If the liquid has a low boiling point it will be volatile, that is it will evaporate easily.
If it evaporates easily then above the liquid will be lots of vapour. If the liquid has a high boiling point it will not be
volatile and there will be very few vapour (gas) particles above the liquid. It is
the amount of
particles in the gas phase above the liquid surface that will determine how flammable it is. When a
lit splint is placed above a flammable liquid; it is not the liquid but the vapour above the liquid
which burns.
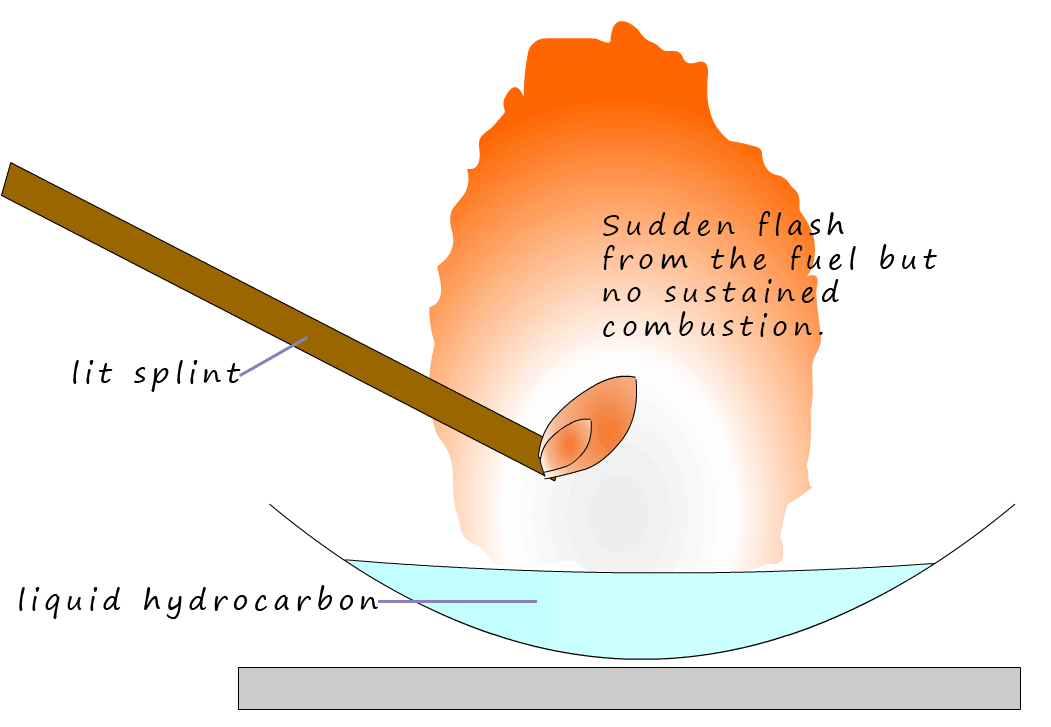
You may hear on the TV drama show CSI or fire fighters talking about the flash point of a substance. The flash point is the point at which there is just enough vapour present above a liquid that when a flame is applied it will flash up BUT there is not enough vapour to keep the flame going. The substance will not burn. However the fire point is the point at which there is enough vapour present to sustain combustion and the fuel will continue to burn. Roughly speaking the fire point is about 100C higher than the flash point. The lower the flash point the more flammable the fuel will be. The table below gives the flash points for several alkane molecules.
| alkane | methane | propane | pentane | heptane | decane |
|---|---|---|---|---|---|
| flash point/oC | -188 | -104 | -49 | -7 | 46 |
 The trend in the flash points and fire points are very clear, the larger the molecule the higher is its flash
point and hence the less flammable it will be. It is also worth noting that the smaller the
hydrocarbon molecule the more cleanly it burns. Large chain hydrocarbons are much more difficult to
ignite than smaller chain hydrocarbons and they burn with very dirty sooty flames. You may also hear
people refer to incomplete combustion as dirty combustion because of the soot it produces. You can
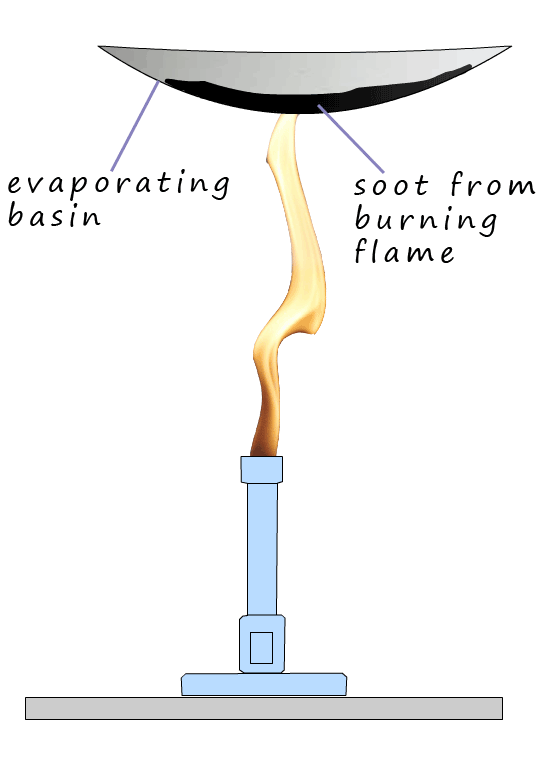
easily see the soot in a flame by placing a cup or in the image opposite a clean white evaporating
basin after a few seconds in the flame, the nice clean evaporating basin is black with soot.
The trend in the flash points and fire points are very clear, the larger the molecule the higher is its flash
point and hence the less flammable it will be. It is also worth noting that the smaller the
hydrocarbon molecule the more cleanly it burns. Large chain hydrocarbons are much more difficult to
ignite than smaller chain hydrocarbons and they burn with very dirty sooty flames. You may also hear
people refer to incomplete combustion as dirty combustion because of the soot it produces. You can
easily see the soot in a flame by placing a cup or in the image opposite a clean white evaporating
basin after a few seconds in the flame, the nice clean evaporating basin is black with soot.