Higher and foundation tiers
The chemical properties of the alkanes
Chemically the alkanes are pretty dull and uninteresting, the alkanes are generally very unreactive molecules, the only real use for them is as fuels.
They are used to fuel the world! In fact alkane molecules provide the fuel for cars, trains, ships, planes and pretty much
any other form of transport that you can think of.
In this page we will discuss the products produced by the combustion of hydrocarbons such as the alkanes. Before looking at the combustion reactions of the alkanes consider the following two combustion reactions:
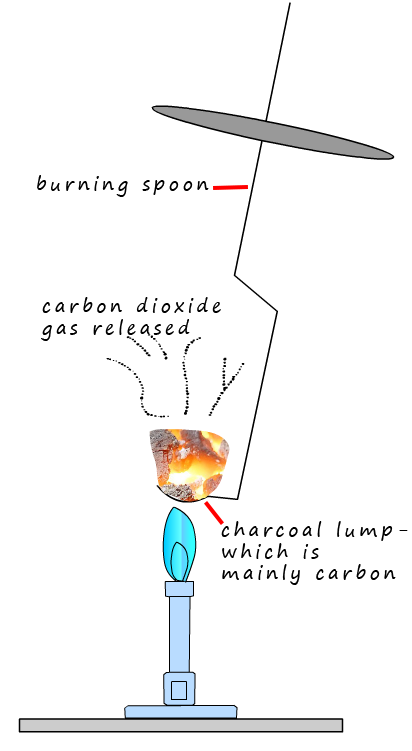
The combustion of carbon
1. Carbon burns in air to produce carbon dioxide gas. The image opposite shows a lump of charcoal; which is mainly carbon burning in air to form carbon dioxide gas.
Equations for this reaction are shown below:
carbon(s) + oxygen(g) → carbon dioxide(g)
C(s) + O2(g) → CO2(g)
The combustion of hydrogen
2.
Hydrogen gas burns in air to produce hydrogen oxide (water).
hydrogen(g) + oxygen(g) → hydrogen oxide(g)
2H2(g) + O2(g) → 2H2O(g)
The combustion of hydrocarbon molecules
Since the alkanes are hydrocarbons
and hydrocarbons contain only the elements carbon and hydrogen,
then
when a hydrocarbon is combusted or burned in air the products should be carbon dioxide
and water. Essentially when you
burn or combust a substance you take each element in turn that is present in the compound and simply join it
with oxygen.
The combustion of methane
3. Methane (CH4) is the gas used in Bunsen burners and at home for cooking and heating. It is also a hydrocarbon; that is it is a compound which contains only the elements carbon and
hydrogen, so when it combusted in air it will produce carbon dioxide and hydrogen oxide (water vapour):
methane(g) + oxygen(g) → carbon dioxide(g) + hydrogen oxide(g)
CH4 + 2O2(g) → CO2(g) + 2H2O(g)
Complete and incomplete combustion
The gas we use at home for cooking and to heating is methane. Methane is also used in
the science lab as the fuel for Bunsen burners. If burning the hydrocarbon methane forms carbon dioxide and water vapour then burning any hydrocarbon will also produce the same carbon dioxide and water vapour as the products, for example the hydrocarbon pentane (C5H12) burns or combusts according to the equation below:
pentane(g) + oxygen(g) → carbon dioxide(g) + hydrogen oxide(g)
C5H12 + 8O2(g) → 5CO2(g) + 6H2O(g)
The only difference between burning pentane and methane is that because pentane is a larger molecule which contains more carbon and hydrogen atoms it requires more oxygen to burn or combust completely and it will also produce more water vapour and more carbon dioxide gas.
Sometimes this can be a problem, for example if you try to burn a fuel inside sealed containers such as engines or a boiler where there maybe be a lack of oxygen.
Burning hydrocarbons in air where there is plenty of oxygen available results in the production of carbon dioxide gas and water vapour. This is called complete combustion.
Burning hydrocarbons where there is a limited supply of oxygen is called incomplete combustion and different products are obtained. These reactions also release less energy than the complete combustion reactions e.g. the two equations below show the products produced by the complete and incomplete combustion of the hydrocarbon molecule pentane.
C5H12 + 8O2(g) → 5CO2(g) + 6H2O(g) complete combustion of pentane
C5H12 + 51/2O2(g) → 5CO(g) + 6H2O(g) incomplete combustion of pentane
For the complete combustion of pentane 8 moles of oxygen gas are required for every mole of pentane burned, however if pentane is burned in an atmosphere containing a limited supply of oxygen gas then the poisonous gas carbon monoxide (CO) is
produced instead of carbon dioxide.
The complete and incomplete combustion of methane gas
Methane gas as mentioned is the gas used in Bunsen burners in the science lab. The two common flames used on the Bunsen burner are the safety and the roaring flames. The safety flame is an orange/yellow visible flame produced when the air hole on the Bunsen burner is closed while the roaring flame is produced when the air hole on the Bunsen burner is fully opened. Opening the air hole allows air to be drawn into the Bunsen chimney where it mixes with the methane gas. This means that when the mixture of air and methane are lit at the top of the chimney there is enough oxygen present in the mixture of gases for complete combustion to take place.
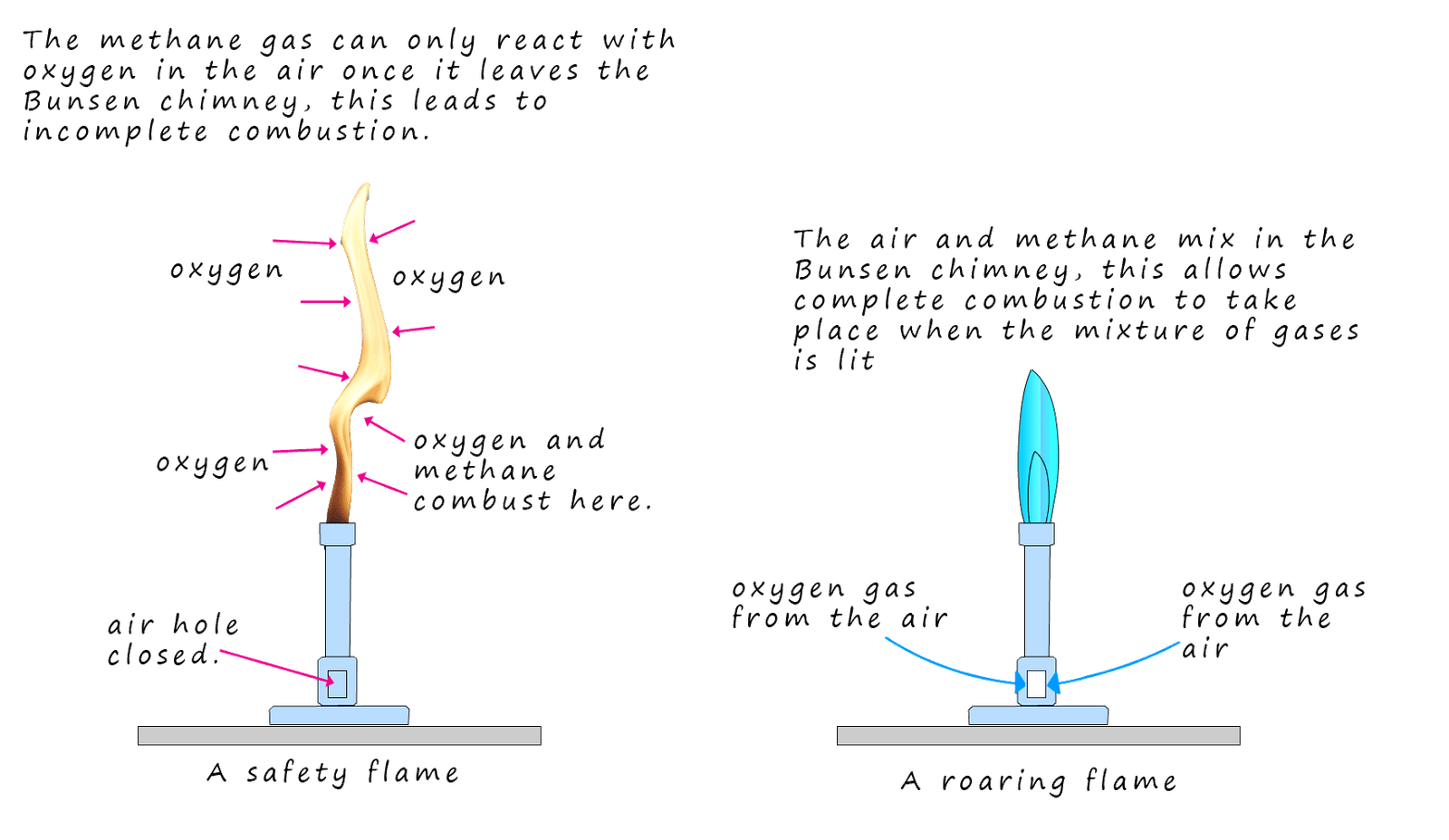
However if the air hole on the Bunsen burner is closed then methane gas can only combust once leaves the chimney and mixes with the air. This leads to incomplete combustion simply because there is not enough oxygen for the methane to react with. The luminous safety flame produced by this incomplete combustion reaction is due to the presence of soot particles in the flame, these soot particles reflect light making the flame visible. This is outlined in the image shown.
The word and symbolic equations for these combustion reactions of methane are given below:
CH4 + 2O2(g) → CO2(g) + 2H2O(g) complete combustion of methane
CH4 + 11/2O2(g) → CO(g) + 2H2O(g) incomplete combustion of methane
The incomplete combustion of methane produces carbon monoxide whereas when methane is burned in a plentiful supply of oxygen produces
carbon dioxide gas.
So where does the soot come from in the luminous Bunsen flame? Well keep reading for the answer......
Burning propane gas
Propane (C3H8) is another commonly used alkane. Propane gas (C3H8) is sold in gas bottles for use in BBQs, heating homes and caravans; it is also used in the construction industry. The equations below show the products of the complete and incomplete combustion of propane gas.
C3H8 + 5O2(g) → 3CO2(g) + 4H2O(g) complete combustion of propane
C3H8 + 31/2O2(g) → 3CO(g) + 4H2O(g) incomplete combustion of propane
 It is a similar story when propane is burned; the complete combustion of a hydrocarbon fuel like propane produces carbon dioxide and water vapour as
the products whereas the incomplete combustion produces the toxic gas carbon monoxide and water vapour.
It is a similar story when propane is burned; the complete combustion of a hydrocarbon fuel like propane produces carbon dioxide and water vapour as
the products whereas the incomplete combustion produces the toxic gas carbon monoxide and water vapour.
Note, in some of the equations above; for example in the incomplete combustion of methane and propane I have used 11/2 and 31/2 moles of oxygen to
balance these equations. In some textbooks you may see that instead of having decimals or fractions in equations they simply double the number of moles in the equations.
This will remove the decimals or fractions in the equations however the choice is yours as to whether to leave them as I have written the equations or double everything to remove the fractions!
 If the hydrocarbons are burned in a very limited supply of air/oxygen it is possible that no carbon monoxide
will be produced; instead solid carbon or soot is produced e.g. The equations below show the equations for the complete and incomplete combustion of propane gas (C3H8) when various amounts of oxygen are available.
If the hydrocarbons are burned in a very limited supply of air/oxygen it is possible that no carbon monoxide
will be produced; instead solid carbon or soot is produced e.g. The equations below show the equations for the complete and incomplete combustion of propane gas (C3H8) when various amounts of oxygen are available.
propane + oxygen → carbon (soot) + hydrogen oxide incomplete combustion of propane
C3H8 + 3O2(g) → 3CO(g) + 4H2O(g) incomplete combustion of propane
C3H8 + 2O2(g) → 3C(s) + 4H2O(g) incomplete combustion of propane
(Hint- To balance these equations,
however many carbon atoms are in the hydrocarbon that is the number of moles of soot produced,
however many moles of hydrogen are in the hydrocarbon half this number of moles of water will be
produced).
It is very likely that when the hydrocarbon fuels undergo combustion that a
combination of carbon dioxide, carbon monoxide and soot will all be produced. It all depends on the amount of oxygen gas available when the fuel is burned. The equations below show the complete
and incomplete combustion of hexane (C6H14):
hexane + oxygen → carbon (soot) + hydrogen oxide incomplete combustion of hexane
C6H14 + 61/2O2(g) → 6CO(g) + 7H2O(g) incomplete combustion of hexane
C6H14 + 31/2O2(g) → 6C(s) + 7H2O(g) incomplete combustion of hexane
Testing for the products of combustion
As discussed above the products for the complete combustion of a hydrocarbon are carbon dioxide
and water. The apparatus below can be set-up to collect the products of combustion of a hydrocarbon and to test
them.
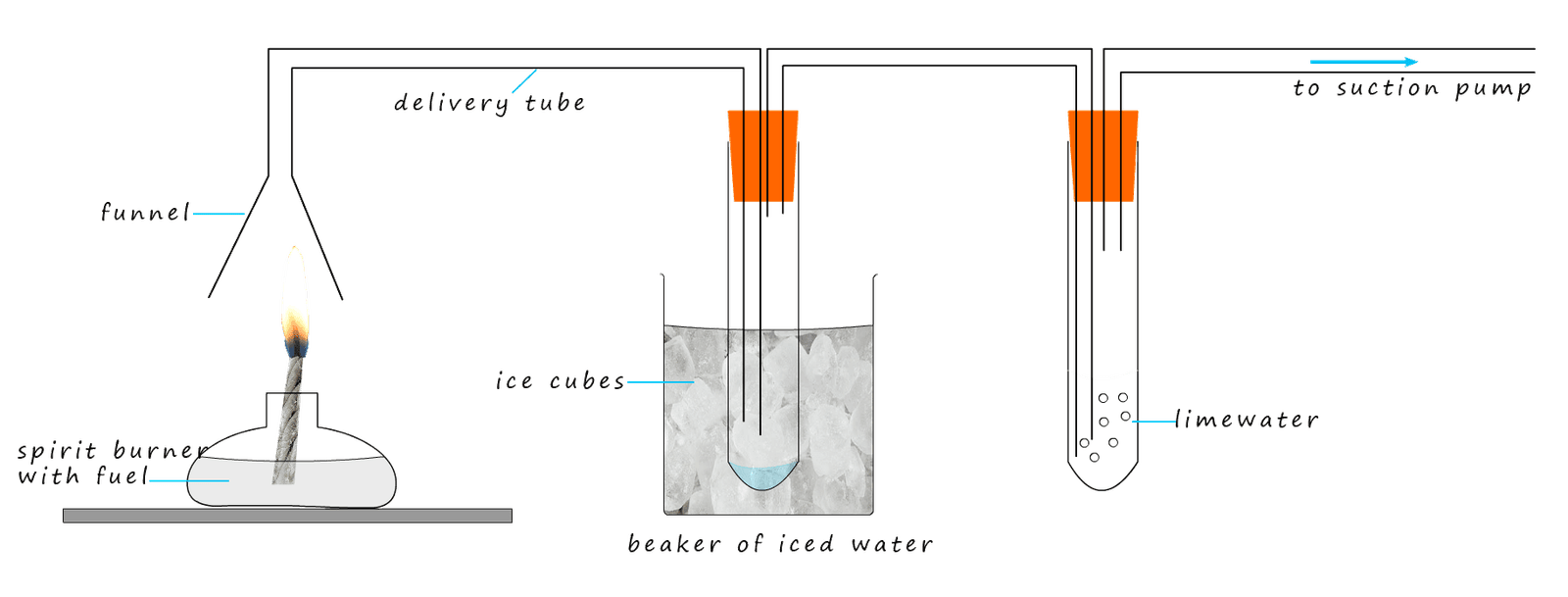
The products of complete combustion of hydrocarbon fuel are carbon dioxide and water vapour; these two gases leave the candle flame and are drawn into the funnel by a suction pump.
Here the vapours enter the first boiling tube. This boiling tube is surrounded by ice chilled
water, this will cause the water vapour from the combustion reaction to condense and collect as liquid water
inside the boiling tube. This can then be tested with blue cobalt chloride paper (test for water-
blue cobalt chloride paper turns pink). Alternatively the boiling tube can be filled with anhydrous copper sulfate. This is a white solid which turns blue when water is added to it. The carbon dioxide from the combustion reaction will pass through this
first test-tube and enter the second boiling tube. This tube is filled with limewater. The
limewater will turn chalky or milky due to the presence of carbon dioxide.
As mentioned above the main use for alkanes is as fuels. Many fuels not only contain alkanes but
also the element sulfur. Sulfur is a yellow solid in its elemental form. It forms an acidic non-metal
oxide when it is burned:
sulfur(s) + oxygen(g) → sulfur dioxide(g)
S(s) + O2(g) → SO2(g)
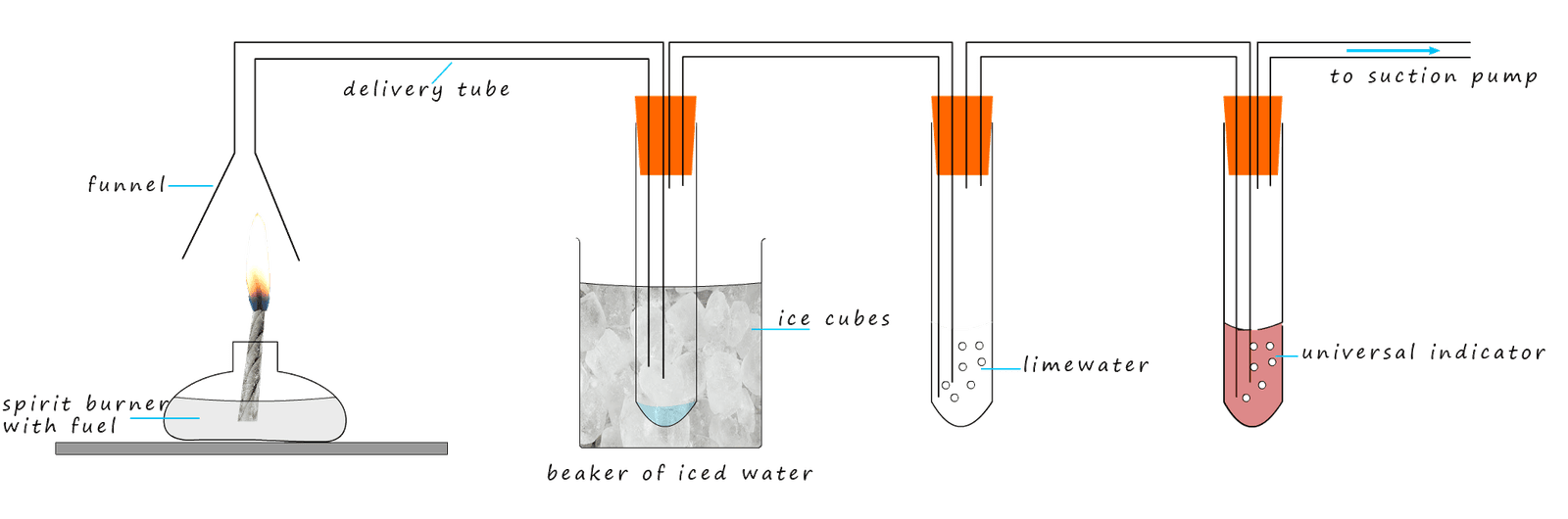
Sulfur dioxide gas being an acidic gas will dissolve in water to form an acid; sulfurous acid. We can modify the apparatus
set-up above to test for an acidic gas by simply adding another boiling tube filled with universal indicator solution.
If any acidic gases are present; such as sulfur dioxide then the universal indicator solution will turn red. This new set-up is shown below:
Key Points
- When a compound is burned in air each of the elements in the compound will form an oxide.
- Carbon will completely combust to form carbon dioxide. Hydrogen will combust to form hydrogen oxide
or water. Since hydrocarbons contain only the elements carbon and hydrogen they will under complete combustion to form carbon dioxide and water vapour.
- Complete combustion occurs when a fuel is burned in a plentiful supply of air (oxygen). The products
of the complete combustion of an alkane (hydrocarbon) are CO2 and H2O. Incomplete combustion
occurs when fuels are burned in a limited supply of air (oxygen); for example inside car engines and gas boilers. The
products of the incomplete combustion of a hydrocarbon fuel are likely to be a mixture of substances such as: carbon dioxide, carbon monoxide, soot (carbon)
and water vapour.
- Blue cobalt chloride paper is used to test for water; it turns pink in water. Limewater is used to test for
carbon dioxide; it turns milky or chalky when carbon dioxide gas is bubbled through it.
Practice questions
Next




 It is a similar story when propane is burned; the complete combustion of a hydrocarbon fuel like propane produces carbon dioxide and water vapour as
the products whereas the incomplete combustion produces the toxic gas carbon monoxide and water vapour.
It is a similar story when propane is burned; the complete combustion of a hydrocarbon fuel like propane produces carbon dioxide and water vapour as
the products whereas the incomplete combustion produces the toxic gas carbon monoxide and water vapour.
 If the hydrocarbons are burned in a very limited supply of air/oxygen it is possible that no carbon monoxide
will be produced; instead solid carbon or soot is produced e.g. The equations below show the equations for the complete and incomplete combustion of propane gas (C3H8) when various amounts of oxygen are available.
If the hydrocarbons are burned in a very limited supply of air/oxygen it is possible that no carbon monoxide
will be produced; instead solid carbon or soot is produced e.g. The equations below show the equations for the complete and incomplete combustion of propane gas (C3H8) when various amounts of oxygen are available.
