
Chemistry only
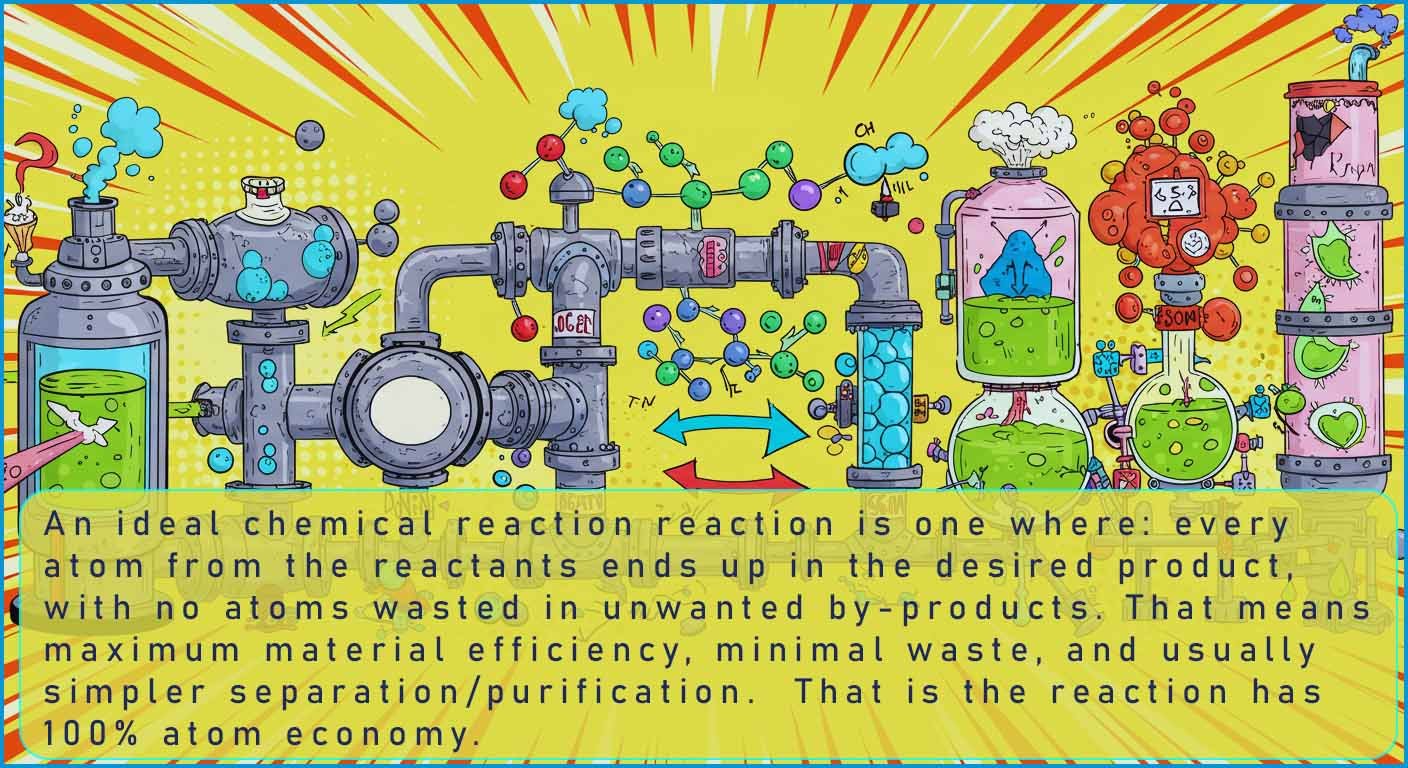
The image below summarises what an ideal chemical reaction might look like:
Let's suppose that a chemical company intends to start manufacturing a new product which they hope will to bring to market and sell to increase their profits, let's call this new product; product C. Now the chemists working for this particular chemical company have come up with two separate methods to make this new product C; these two methods are shown below:
So which method would you choose to make the new product C? Which method do you think will be the most profitable, sustainable and environmentally friendly? Let's look at some of the advantages and disadvantages for each of the proposed methods.
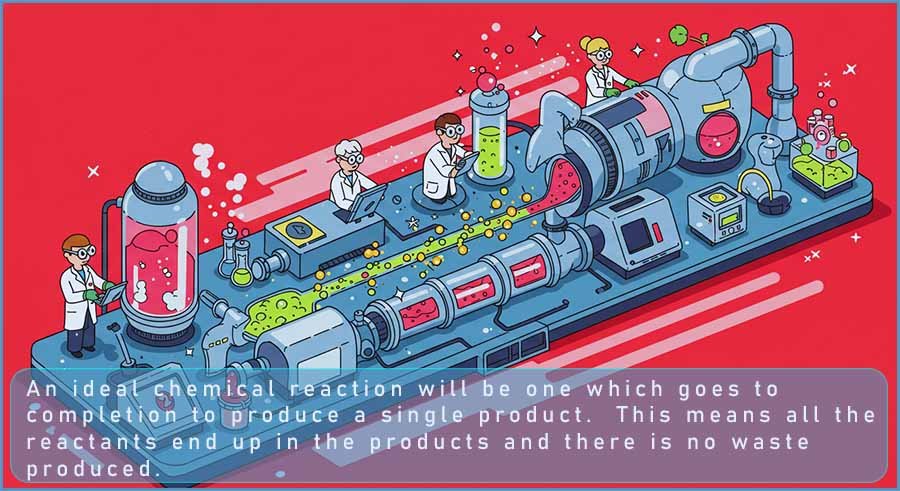 Method 1 has one major advantage over method 2, method 1 only produces the desired product C.
This means that all of the reactants are going into making the desired product and no other unwanted products are formed. This method also goes to completion, the arrow (→) used in the equation indicated that ALL of the reactants are turned into the desired product C. This is excellent since if there is only one product formed then there will be no expense incurred as a result of separation issues which would be the case if the reaction produced a mixture of two or more products. There is also no waste to be disposed of, which is likely to be both expensive and also environmentally unfriendly to dispose of.
Method 1 has one major advantage over method 2, method 1 only produces the desired product C.
This means that all of the reactants are going into making the desired product and no other unwanted products are formed. This method also goes to completion, the arrow (→) used in the equation indicated that ALL of the reactants are turned into the desired product C. This is excellent since if there is only one product formed then there will be no expense incurred as a result of separation issues which would be the case if the reaction produced a mixture of two or more products. There is also no waste to be disposed of, which is likely to be both expensive and also environmentally unfriendly to dispose of.The goal of any good chemist is to design a process where the maximum amount of the desired product is obtained with as little waste and undesired products as possible being produced. That is the reaction should have a high percentage yield, it ideally should have a reasonable rate of reaction and not involve waiting for days or even longer before any reasonable amount of the desired product is produced.
Reactions which are reversible will obviously result an equilibrium mixture being produced which could mean separation issues and additional expense before any of the desired product is obtained. Any waste products produced will have to be disposed of and this will incur costs both financial and environmental. Producing lots of unwanted products or waste materials also uses up valuable natural resources and will cause additional environmental pollution both in their extraction and their disposal; these processes are likely to be unsustainable.
One of the most important considerations in deciding which of the two methods above to use in producing the new product C is the atom economy or atom utilisation for each method. The atom economy or atom utilisation is used as a measure of how much of the reactants end up in the desired product; it can be calculated using the formula shown in the box below:

The higher the value of the atom economy the more of the reactants end up in the desired product, while any chemical reaction with a low atom economy is one where a large amount of the reactants end up in unwanted or waste products, this means that large amounts of reactants are likely to be need to manufacture small amounts of the desired product and lots of waste products will be formed; this is unlikely to be profitable, environmentally friendly and certainly not sustainable.

As an example consider the fermentation of glucose to form the alcohol ethanol and carbon dioxide gas, word and symbolic equations for this reaction are shown below:
Now the relative formula masses of all the reactants and products are:
The fermentation process has a relatively low atom economy of just over 51%, meaning that just over half of the mass of the reactants ends up in the desired product; the ethanol. Improving the atom economy of this reaction is very desirable in order to reduce the amount of waste or undesired product and make the process more sustainable and cost-effective.
Review your understanding of atom economy by completing the activity below. Simply click or drag the statements into the true or false bins and click the check answers button when your done.
Drag items into the bins or click an item, then click a bin.
The relative formula masses for all the reactants and products are:
The reaction shown to make ammonia is a reversible reaction and so will involve an equilibrium mixture of products and reactants; it just so happens in this particular reaction that the position of equilibrium lies very much to the left, that is in favour of the reactants. Finally the rate of the forward reaction is also very slow due to the fact that it has a large activation energy. So while atom economy is very important it is not the only factor that should be considered in deciding whether a reaction will be profitable, sustainable and environmentally friendly.
Calculate the atom economy for the reactions in the questions below; press the check answer button when you are done.
Question: Calculate the atom economy (%) for the desired product in the equation below. Use the given Ar values. Give your answer to 1 decimal place.
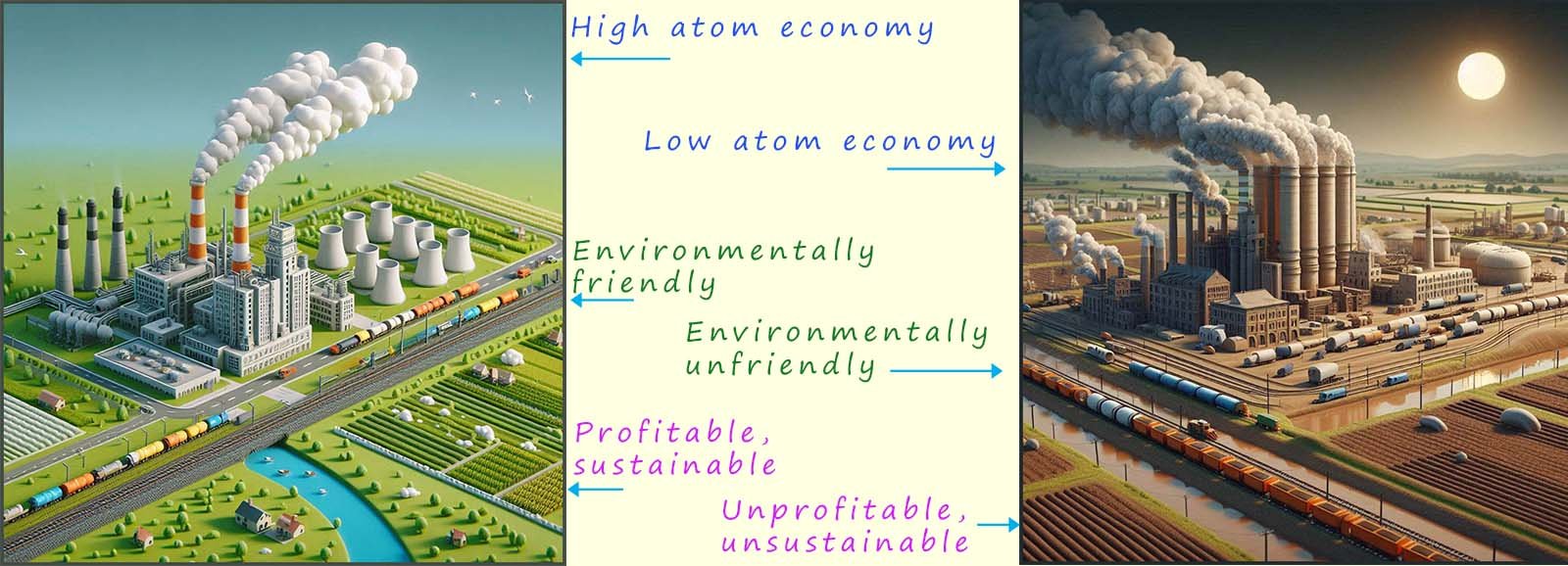
The image below shows two different chemical factories. One factory utilises reactions with high atom economies which produce little waste and which cause low levels of pollution; this factory also utilises all of the raw materials it consumes and so is sustainable. The other chemical factory does none of these things!!