

Chemistry only
Ceramic materials are all around us. Bricks and other clay-based ceramics probably make up much of the building you are sitting in. You may even be drinking from a ceramic mug🍵 or a glass tumbler🥛 while using your computer or laptop, which itself contains ceramic insulators and semiconductor components. Most people, when they hear the word ceramics, think of simple items such as mugs, cups and bricks🧱. However, ceramics include a much wider range of materials with many important uses.
Ceramics can be thought of as non-metallic, solid materials made by heating naturally occurring raw materials to very high temperatures. Ceramics contain metal and non-metals which combine to form either giant covalent structures or giant ionic lattices. They can be broadly divided into three main groups:
| Ceramic Material | Properties | Typical Uses |
|---|---|---|
| Zirconia (ZrO₂) 🔪 | Very hard, tough, wear-resistant, non-porous | Knife blades, dental crowns, hip joints, fuel cell parts |
| Alumina (Al₂O₃) ⚡ | High melting point, electrical insulator, chemically resistant | Spark plug insulators, laboratory crucibles, cutting tools, implants |
| Silicon carbide (SiC) 🛠️ | Extremely hard, heat-resistant, abrasion-resistant | Saw blades, grinding wheels, furnace linings, brake discs |
| Silicon nitride (Si₃N₄) 🚗 | Very strong, lightweight, resists thermal shock | Ball bearings, engine parts, aerospace components |
| Boron carbide (B₄C) 🛡️ | One of the hardest materials known, extremely wear-resistant | Body armour, tank armour, abrasives, neutron shields |
| Piezoelectric ceramics 🔊 | Convert pressure into electrical signals and vice-versa | Microphones, ultrasound probes, speakers, sensors |
| Borosilicate glass 🧪 | Low thermal expansion, chemically resistant, tough | Lab glassware, oven dishes, chemical plant pipes, telescopes |
Traditional ceramics are mostly made from clay and other natural minerals. When people think of ceramics, they often picture everyday clay ceramic items such as sinks, toilets, wall and floor tiles, roof tiles and pottery. The images below show some common examples of traditional clay-based ceramic items:
Terracotta🏺, which translates from Italian as "baked earth," is one of the oldest and most fundamental types of earthenware clay ceramic, it is made from common, coarse and highly iron-rich clay that gives it a classic reddish-brown or rust colour when fired. It is distinguished from other types of clay based ceramics like porcelain which is used to make plates, cups and other tableware by its low-temperature firing process, typically between 800 and 1100°C🔥, which prevents the clay from fully vitrifying (turning into a glass-like solid), leaving the finished product porous and water-absorbent, making it ideal for applications like flower pots🪴 and unglazed roofing tiles.
Click the button below to find out about the terracotta army. An army of around 8000 soldiers, chariots and horses made entirely from clay. The terracotta army is probably one of the most significant archaeological discoveries in history.



Perhaps some of the most remarkable items ever made from clay are the Chinese terracotta warriors⚔️ (shown in the images opposite). The terracotta warriors are life-sized clay soldiers that were created to guard the tomb of China’s first emperor, Qin Shi Huang.
They were buried over 2,000 years ago, around 210 BCE near the city of Xi’an in northern China. The buried terracotta army was accidentally discovered in 1974 by farmers digging a well and they accidentially uncovered thousands of clay soldiers, horses and chariots; each one was individually crafted with unique facial features and armour.
The clay used to create these life-sized soldier figures was locally sourced and meticulously crafted. The construction process involved assembling the figures from separate pieces of clay which were then moulded in various sizes and shapes. These clay parts were then fired in large kilns at fairly high temperatures to solidify and preserve them. The soldiers were then painted in bright colours, which have unfortunately for many of the terracotta soldiers faded over the 2000 years in which they remained buried and undiscovered.
The clay used to make bricks 🧱 and roof tiles 🏠 is usually dug from local quarries, it is then ground and screened to remove unwanted substances such as stones and rocks. Water is then added to make the clay soft and mouldable. The wet clay is then forced through a die which creates a long thin rectangular column of wet moist clay, a wire will then simply slice this into individual brick 🧱sized blocks, or roof tile🏠 sized blocks.

Bricks like most ceramics are very strong in compression; imagine the weight pushing down on a single brick at the base of the wall in the image opposite or on a brick at base of a tall industrial chimney. Bricks like ceramics in general also have high resistance to wear and tear. However bricks are brittle; drop one onto a hard surface and it is likely to break. This is one of the main problem with ceramics in general - they are brittle.
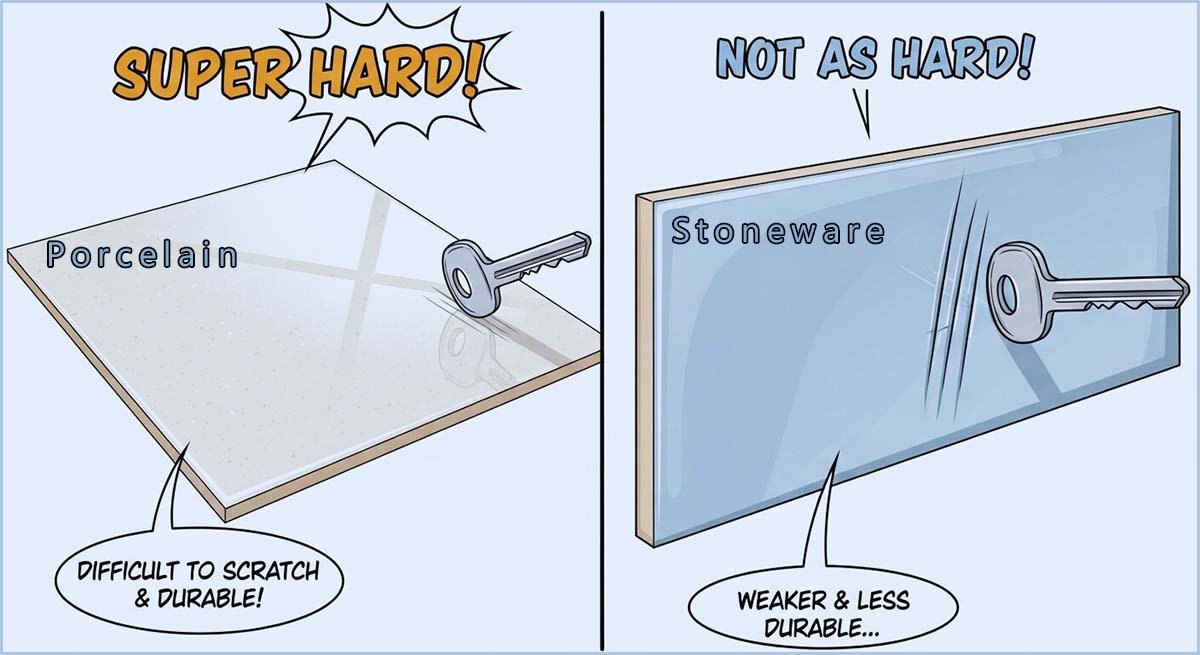
Earthenware, stoneware and porcelain are three types of clay ceramics that differ mainly from each other due to the temperature at which the clay they are made from is fired in the kiln. For example many everyday cups, mugs🍵 and plates 🍽️are made from porcelain or stoneware. The ceramic porcelain is fired at the highest temperatures of the three around 1200–1400°C, it is this high temperature which causes the clay to become almost completely vitrified. This makes porcelain very strong, hard and non-porous, so it will not absorb water or stain. Because of this most fine tea cups, coffee cups and dinner plates; especially the smooth white and high-quality ones are made from porcelain. Porcelain is also the most expensive of the three main ceramic types because it uses highly refined clays, requires very high firing temperatures and needs more careful manufacturing.
Stoneware is fired🔥 at slightly lower temperatures than porcelain; around 1100–1300°C and becomes only partly vitrified. However stoneware is still a strong, tough and chip-resistant ceramic, but it is not as strong or as durable as porcelain. Everyday mugs and heavier plates or bowls are often made from stoneware because it is a durable and almost non-porous ceramic. Stoneware is less expensive than porcelain simply because it is easier to make and fires at lower temperatures, but it is still a high-quality useful ceramic.
Earthenware ceramics are fired🔥 at lower temperatures about 800–1100°C, as a consequence of this low temperature firing🔥 they remains porous unless a glaze is added and earthenware is also weaker and less durable than stoneware and porcelain. Earthenware is used for some inexpensive plates and decorative pottery, but it is less suitable for items that need to withstand regular washing, heat and everyday use. Earthenware is the cheapest of the three ceramics because it uses common clays and requires the lowest firing temperatures🔥. This is summarised in the table below:
| Type of ceramic | Firing temperature | Strength | Porosity | Common uses |
|---|---|---|---|---|
| Earthenware | ~800–1100°C | Low | Porous | Wall tiles, basic pottery, decorative items |
| Stoneware | ~1100–1300°C | Medium–high | Low | Mugs, bowls, cookware, some tiles |
| Porcelain | ~1200–1400°C | Very high | Very low | Floor tiles, high-end tableware, labware |
Wall tiles 🟦 are normally made from standard ceramic ( earthenware) because they do not need to withstand heavy loads or constant wear. Floor tiles ⬜ however must cope with people walking on them, the weight of furniture and regular cleaning so they are usually made from porcelain, which is much harder, stronger and more durable. Although the final properties are different, both wall and floor tiles start with the same basic raw materials: clays such as kaolin, silica (sand), and feldspar. These ingredients are finely ground and then mixed with water to form a thick liquid called a slurry. The slurry is then dried to make a fine powder, which is then compressed in moulds to form the basic tile shape 🟦.
The unfired tiles are dried further to reduce the water content to about 1%; too much moisture can make the tiles warp or crack when they are fired in the kiln. A liquid glaze is then applied. This glaze gives the tiles their colour and pattern and forms a glassy, waterproof surface when heated. The tiles are then fired in a kiln. Wall tiles 🟦 are typically fired at around 1000–1200°C, which gives a hard but slightly porous ceramic. Floor tiles ⬜ are fired at a much higher temperature, around 1200–1400°C. At these temperatures the clay becomes highly vitrified, producing porcelain tiles that are far stronger, denser, more water-resistant and more resistant to scratching and chipping.
Porcelain is also used to make high-quality dinnerware such as plates 🍽️, saucers and cups 🍵 because it is very strong, hard and non-porous. Its high-temperature firing makes it almost completely vitrified, meaning it will not absorb moisture or food stains and can withstand rapid temperature changes, making it safe for microwave ovens and everyday use.

Ceramics have many desirable properties, which explains why they are found in such a wide variety of materials. Silicon carbide for example is an advanced (or technical) ceramic and not a clay-based ceramic. It is used widely for example in cutting and grinding tools because it has a combination of some very desirable properties:
For these reasons silicon carbide is used in masonry saw blades, concrete and brick-cutting discs, grinding wheels and abrasive papers such as sandpaper. In most cutting tools the whole blade is not made from the ceramic; instead silicon carbide particles are bonded to a steel disc which results in a cutting blade with the toughness of steel and the cutting ability of the very hard ceramic silicon carbide.
The hardness and ability of ceramics such as silicon carbide and alumina to resist wear, tear and abrasion is a very desirable property of these materials. However another equally importantant and valuable property of ceramics is there ability to act as refractory materials; that is their ability to act as heat resistant materials.

Ceramics typically have very high melting points and do not soften or melt easily when heated, which is why they are described as refractory materials. This makes them ideal for high-temperature applications where metals or polymers would melt or burn. Many furnaces, kilns and wood-burning stoves are lined with ceramic bricks because these bricks can tolerate intense heat without changing shape. Spacecraft for example also use ceramic heat shields, as these tiles can withstand the extreme temperatures experienced when re-entering the Earth’s atmosphere.
There is also many examples of everyday items take advantage of this high heat resistanceof ceramics. Ovenware, porcelain dishes and ceramic cooking pots can be heated to high temperatures without melting or deforming. In school laboratories, ceramic crucibles can be placed directly into the hottest part of a Bunsen flame because they have a giant macromolecular structure with strong covalent bonding present; this allows them to tolerate very high temperatures. Ceramics are also used in components such as spark plugs and electrical insulators on high-voltage pylons, where they must cope with both high heat and high electrical stress without breaking down.
Some advanced ceramics such as silicon carbide and alumina (aluminium oxide) are even more heat resistant than traditional clay ceramics. These materials are used in industrial furnaces, metal-melting crucibles such as those found in metal working industries, jet engine parts and protective coatings, all because they maintain their strength and do not melt even at temperatures above 1500°C. Their ability to tolerate extreme heat is one of the key reasons ceramics are used in so many demanding applications. More examples of ceramics being used in high temperature environments is outlined below:



Ceramics are excellent thermal insulators because they have low thermal conductivity, meaning heat does not travel through them easily. Their strong chemical bonding and giant structures prevent heat energy from passing quickly from one atom to the next, so many ceramic materials stay cool on the outside even when exposed to very high temperatures inside.
Ceramic wool and ceramic fibres for example are widely used to insulate industrial furnaces, kilns and boilers helping them retain heat and operate more efficiently. These materials can withstand temperatures above 1000°C without melting or breaking down. Car exhaust systems also use ceramic insulation as a heat shield to stop heat spreading to nearby components and damaging them.
Ceramics are used in everyday insulation as well. Oven doors often contain ceramic glass, which allows you to see inside while preventing most of the heat from escaping. Ceramic tiles are commonly used to protect kitchen walls from heat generated by cookers, and many electric heaters and toasters use ceramic linings or ceramic supports around heating elements.
Some advanced ceramics, such as alumina and zirconia are used in spacecraft insulation and high-temperature sensors where materials must cope with extreme heat while protecting sensitive equipment. Ceramics with their ability to withstand intense temperatures and provide excellent thermal insulation makes ceramics essential in many modern technologies.



Ceramics are also highly resistant to corrosion and chemical attack because their atoms are held together by strong covalent or ionic bonds that do not react easily with acids, alkalis or most solvents. This makes ceramics ideal for harsh chemical environments where metals would rust, corrode or dissolve. Now glass is also considered as a type of ceramic because it is made from similar raw materials such silica/sand and metal oxides and it is produced by heating these materials to very high temperatures before cooling them to form solid glass.
One major difference between glass and the ceramic materials is most ceramics are crystalline, that is they have ordered structures whereas glass has an amorphous or disorganised structure (this is covered in more detail on the page on glass, its properties and structure). Borosilicate glass for example is used to make laboratory beakers, test tubes and measuring cylinders because it can withstand strong acids, alkalis and rapid temperature changes without cracking.
Many chemical reaction vessels, storage tanks and industrial pipes are lined with ceramic materials to prevent corrosion from hot, corrosive liquids. Ceramics are also used inside chemical processing plants, desalination equipment and wastewater-treatment systems where long-term resistance to chemicals is essential.
In the human body dental implants, crowns and bridges make use of ceramics because they do not react with saliva or acids in food and they remain chemically stable for decades. Joint replacements also contain ceramic components that resist corrosion from bodily fluids far better than metals.
Many ceramics are excellent electrical insulators because they do not contain free electrons or mobile ions. This property makes them extremely useful in electrical and electronic applications. Porcelain and alumina are used on high-voltage pylons to stop the electricity travelling down the metal pylon and causing dangerous short circuits. In car engines, ceramic insulators form part of the spark plugs allowing a powerful electrical spark to fire the fuel mixture without any electricity leaking away. Household electrical equipment also contains ceramic components such as the insulating bases of light bulbs, supports for heating elements in kettles and toasters, and fuse bodies. Some advanced electronic devices such as mobile phones and computers or laptops also use ceramics as substrates on circuit boards because they provide excellent electrical insulation and can withstand high temperatures where plastic insulators would melt.
The examples above discuss some very desirable properties of ceramics; however its not all all good news for ceramics, many ceramics have some less than desirable properties, for example:
If it was possible to manufacture ceramic materials free of these defects then we would have a new wonder material! The good news is that many of the problems associated with ceramics can be partly solved by using them in composite materials.
Test your understanding of the properties of ceramics by completing the activity below:
Click a property on the left, then click the description on the right that you think matches it.
Properties
Descriptions
Take the quick quick below to test your understanding of the properties of ceramics, simply click the button below to start the quiz:
Click True or False for each statement. You can try again if you get one wrong.
1. The Terracotta Army figures are made from clay ceramics that were shaped and then fired in kilns.
2. Most ceramics bend easily before they break, just like metals.
3. Many ceramics are good electrical and thermal insulators.
4. Ceramics usually have low melting points, so they soften easily when heated.
5. Window glass is hard, brittle and a good electrical insulator.
6. Advanced ceramics can be used in medical implants such as hip replacements or dental crowns.
7. Ceramics are often used to line kilns and furnaces because they can withstand very high temperatures.
8. Metals are generally better electrical insulators than ceramics.