
Foundation and higher tiers
Substances containing covalent bonds usually have simple molecular structures, however the non-metal elements carbon and silicon in group 4 of the periodic table not only form substances with small molecular structures but they can also form substances with giant covalent structures).
Some common examples of covalent substances with small molecular structures are shown in the table below:
| Covalent molecule | Molecule | Molecular formula | Description |
|---|---|---|---|
| carbon dioxide | 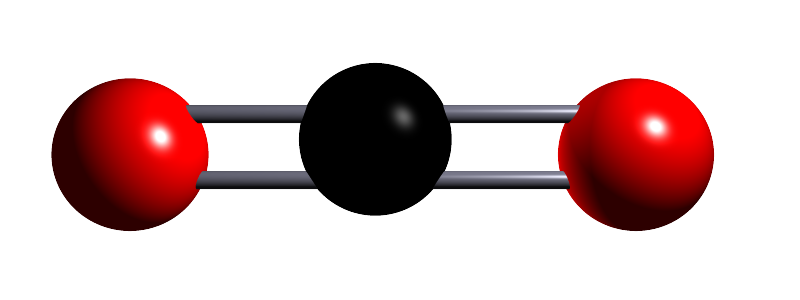 |
CO2 | Small covalent molecule containing one carbon atom and two oxygen atoms. Held together by a double covalent bond between the carbon atom and oxygen atoms. |
| hydrogen oxide (water) | 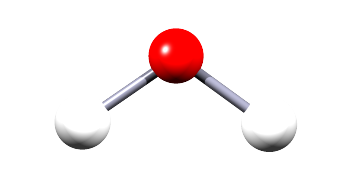 |
H2O | Small covalent molecule containing one atom of oxygen and two atoms of hydrogen. All the present bonds are single covalent bonds. |
| ammonia | 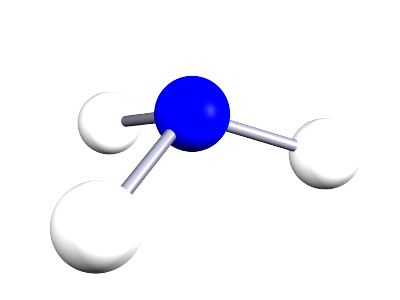 |
NH3 | Small covalent molecule containing one atom of nitrogen and three atoms of hydrogen. All bonds are single covalent bonds. |
| carbon tetrachloride | 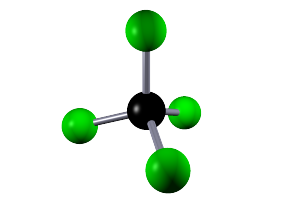 |
CCl4 | Small covalent molecule containing one atom of carbon and four atoms of chlorine. All bonds are single covalent bonds. |
| oxygen | 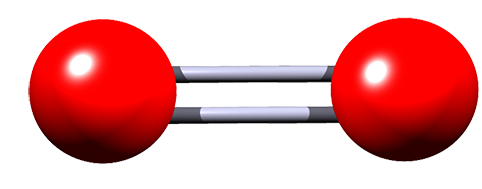 |
O2 | Small covalent molecule containing two atoms of oxygen, held together by a double covalent bond. |
| butane |  |
C4H10 | Small covalent molecule containing four atoms of carbon and ten atoms of hydrogen. All bonds are single covalent bonds. |
| sulfur hexafluoride | 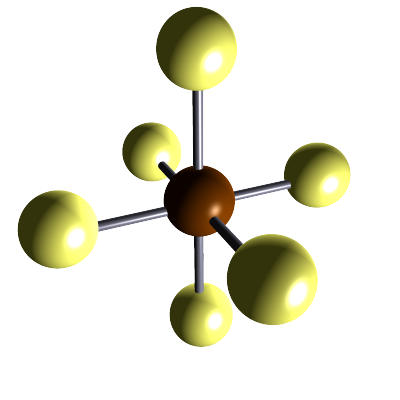 |
SF6 | Small covalent molecule containing one atom of sulfur and six atoms of fluorine. All bonds are single covalent bonds. |
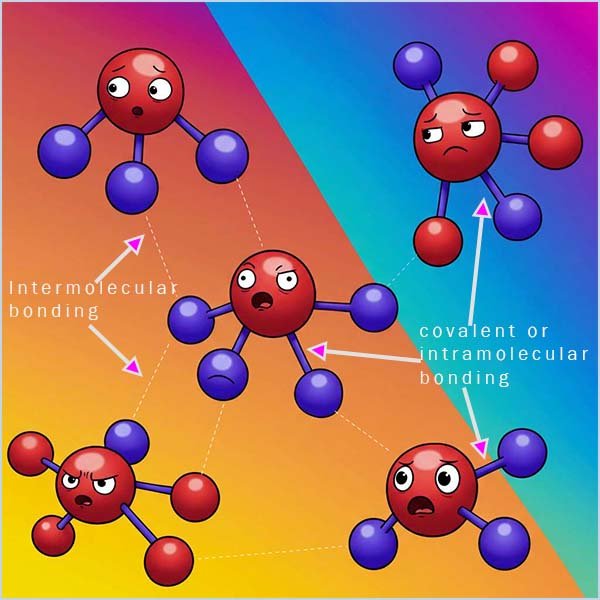
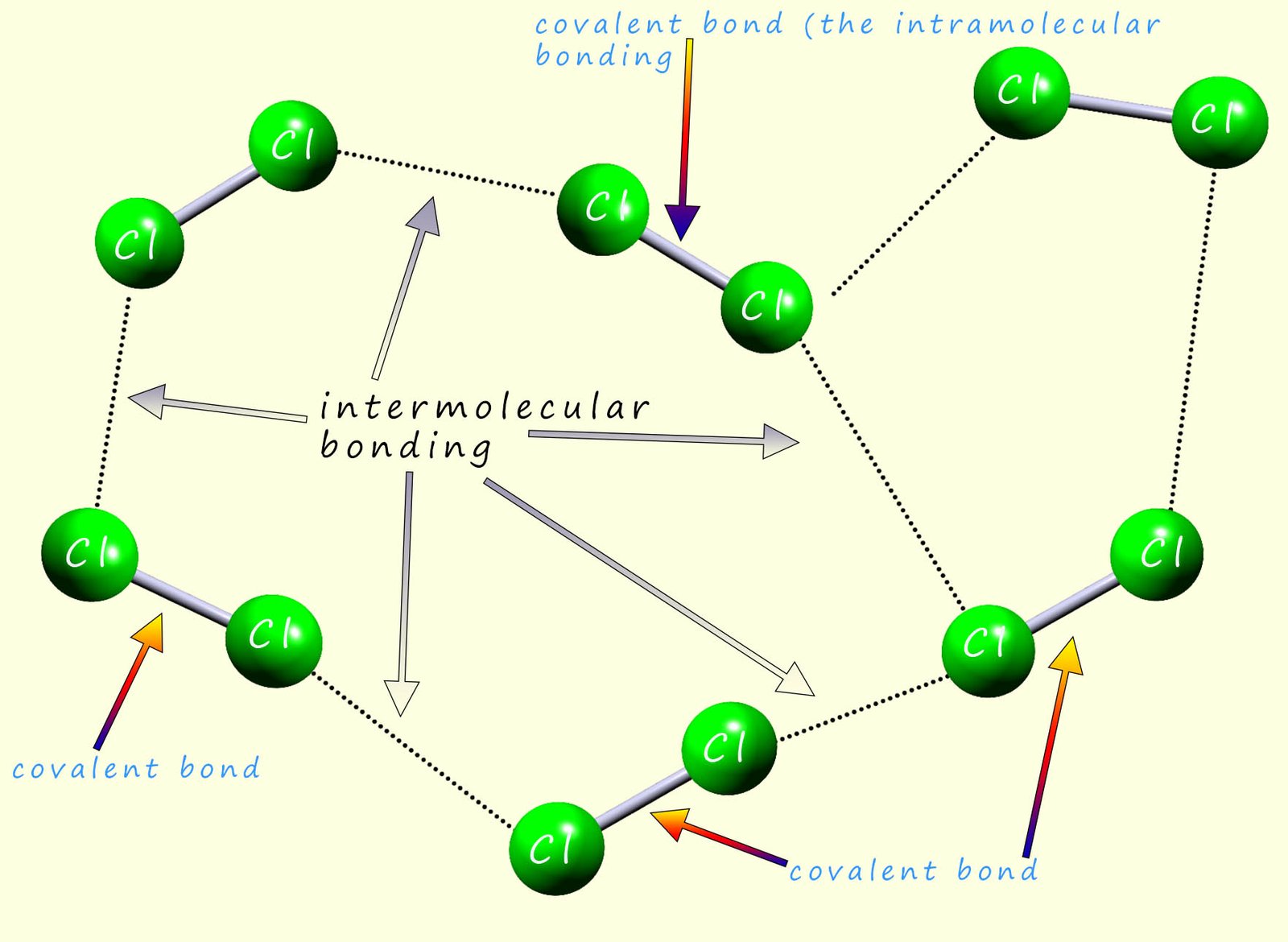
When we consider many of the physical properties of molecular substances such as melting and boiling points you
need to take into account two types of bonding that will be present. In the example below there are six chlorine molecules (Cl2); each
molecule consists of two chlorine atoms which are held together by a single
covalent bond. These covalent bonds
are very
strong and it requires a large amount of energy to break them. This covalent bonding which holds the atoms together in the
molecule is often called the
intramolecular bonding (intra means within).
However as shown in the diagram below as the chlorine molecules get close to each other
there are attractive forces
called Van der Waals forces or London dispersion forces which come into play. These attractive forces between different
chlorine molecules are very weak
when compared to the strength of the covalent
bond between the chlorine atoms (the intramolecular bonding).
These attractive forces between different molecules
are called intermolecular bonds (think of an international as a competition
between different countries, so intermolecular bonding is bonding between different molecules). The strength of these
weak intermolecular bonds increases as the number of electrons
in the atoms increases and as the atoms get larger, however they are always much weaker than a covalent bond. Larger molecules have much more intermolecular bonding than
smaller molecules.
Despite being weak this intermolecular bonding can have a dramatic effect on many of the physical properties of covalent substances. As an example let's look at the melting and boiling points of the halogens in group 7 of the periodic table.
| Halogen | Diameter of atoms in picometres (1x10-12) | Molecule(not to scale) | Molecular formula | Melting point/0C | Boiling point/0C |
|---|---|---|---|---|---|
| Fluorine | 147 |  |
F2 | -219.6 | -188.1 |
| Chlorine | 175 |  |
Cl2 | -101.5 | -34.6 |
| Bromine | 185 |  |
Br2 | -7.2 | 58.5 |
| Iodine | 198 |  |
l2 | 113.7 | 184.3 |
The general trends in the melting and boiling points of the halogens is rather obvious and very dramatic. This trend mirrors the size of the molecules. All of the halogens from fluorine to iodine consist of small diatomic molecules; but obviously the molecules get much larger as we go from the very small fluorine molecules to the much larger iodine atoms.
Small molecules like fluorine have very low melting and boiling points and larger molecules such as iodine have significantly higher melting and boiling points. The molecules are of course getting more massive (heavier) from fluorine to iodine but this would not account alone for such a difference in the melting and boiling points. The reason for the increase in the melting and boiling points is also due to an increase in the amount and the strength of the intermolecular bonding between the molecules - remember the larger the molecule and the more electrons it has the greater the amount of intermolecular bonding present and also the strength of the bonding will be much larger too. So the larger iodine molecules will have much more intermolecular bonding between them than the much smaller fluorine molecules.
Obviously as the size of the molecules increases then the surface area of each molecule will increase and so the area available for intermolecular bonding between the molecules will increase, this is shown below where the larger butane molecules have more intermolecular bonding between them than the smaller ethane molecules.
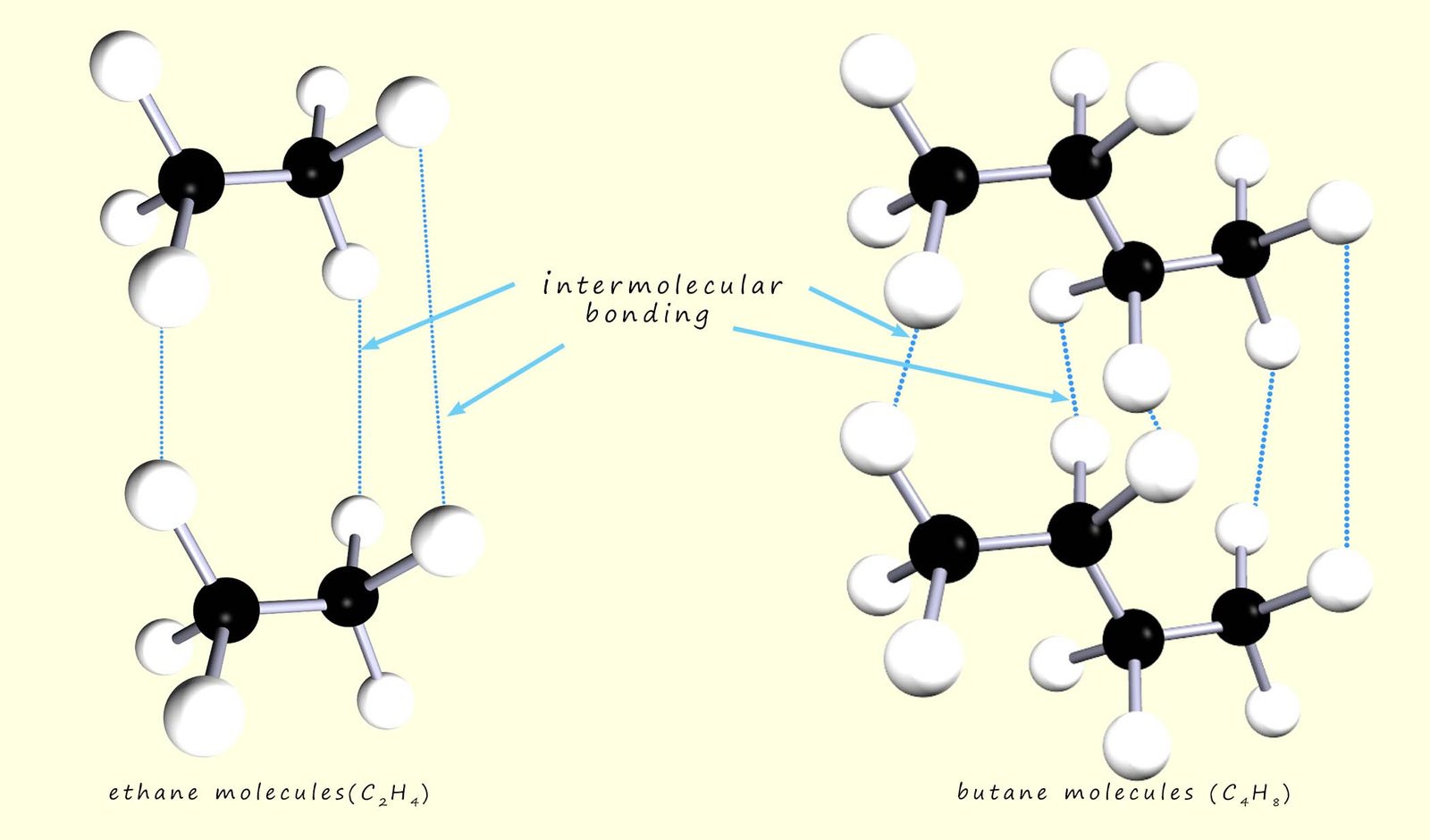
Ethane is a hydrocarbon molecule; its molecular formula is C2H6. Butane is also a hydrocarbon but is a larger molecule; its molecular formula is C4H10. Being larger means butane will have a larger surface area and so when two butane molecules get close to each other there will be more and also stronger intermolecular bonding than you would find between ethane molecules.

This means that melting points and boiling points of butane will be much higher than expected. This is due to the
increased strength of the intermolecular bonding which means more
energy will be needed to separate the molecules.
Remember that when substances change state; whether it is melting or boiling the
covalent bonds never break, it is
only the weak intermolecular bonds that break. Obviously when you try to melt or evaporate a substance
then the molecules in
that substance need to be separated from each other and if there are forces present between these molecules; however weak; holding the
molecules
together and preventing them from being separated then more energy
will be needed to pull them apart from each
other and the result is that the melting and boiling points will be raised.
Despite the presence of intermolecular bonding most
covalent substances still consist of small molecules and so
will be mainly gases and liquids at room temperature. Those that are solids tend to have
low melting points and
some will even sublime on heating.
Iodine is a soft shiny and flaky looking solid at room temperature.
Iodine is a halogen found close to the bottom
of group 7 in the periodic table, it has a molecular structure
consisting of small diatomic molecules and its molecular
 formula is I2. The image opposite shows an iodine molecule.
formula is I2. The image opposite shows an iodine molecule.
From the discussions we have had on the structure of small molecular substances you might expect such a small molecule to be either a liquid or a gas. However at room temperature iodine is a solid and has a macromolecular structure as shown in the image below. Its structure is crystalline (very ordered) and from the model in the image below we can clearly see that solid iodine consists of lots of small diatomic molecules of iodine packed tightly together. The iodine molecules are held together by very covalent bonds (the intramolecular bonding is covalent) but in solid iodine the small diatomic iodine molecules are held in place by weak intermolecular bonds.
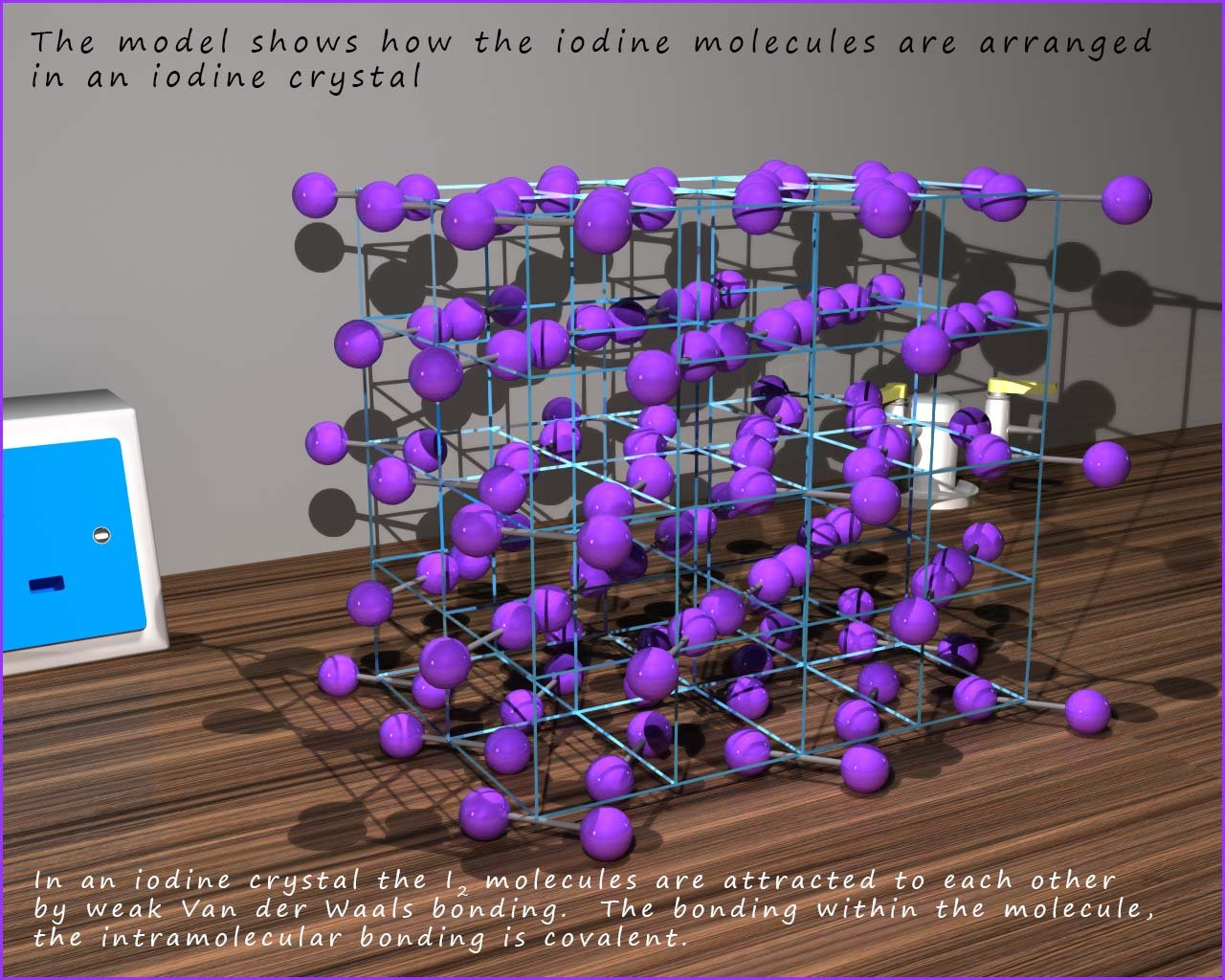
The iodine molecules maybe held together by
strong covalent bonds (the intramolecular bonding)
but in the
macromolecular structure of solid iodine the iodine molecules
themselves are attracted to each other only weakly, that is there is weak
intermolecular bonding between different
molecules. This weak
intermolecular bonding is strong enough to hold the
molecules within the giant structure but if a small amount of
iodine is placed in a beaker sitting in
hot water purple
fumes of iodine vapour are visible above the solid iodine.
Solid iodine when heated gently does not melt; instead it
turn straight from a solid to a vapour (gas) missing out the liquid state completely. This change;
solid to gas is
called sublimation. It happens because
only a small amount of energy is needed to break
the weak intermolecular bonds
between different iodine molecule and these small
diatomic molecules then leave the solid macromolecular structure and
exist simply as individual small I2 molecules in the gaseous state.
The image below shows what happens to the iodine molecules as a crystal of iodine is heated. You can clearly see the individual I2 molecules leaving the macromolecular structure once they gain enough energy to overcome the weak intermolecular bonding holding them in place. You should also see that the covalent bonds within the molecules do not break when the iodine crystal is heated.

Finally most covalent substances do not conduct electricity (except graphite and a few other allotropes of carbon), you probably guessed this as there are no free electrons within the structure, either giant structure or small molecules. All the outer shell electrons are tied up in covalent bonds and none are delocalised or free to move.