

Chemistry only
Corrosion prevention basically falls into 3 categories:

Barrier methods of preventing corrosion rely on some sort of
barrier to block air and/or
water; which are both necessary for
corrosion. For example cars
and bikes are both painted which blocks both air and water
from the surface of the metal. Fences are often
plastic coated
which again puts up a barrier to air and water.
The problem with some barrier methods are once the
barrier is broken then
the metal will corrode.
Painting and plastic coating are also not suitable for moving metal parts e.g. motorcycle and bike chains, engines and tools such as spanners,
hammers etc. Here the moving parts maybe covered with a thin layer of oil or grease when they are not in use to help prevent corrosion.
Electroplated objects are metal objects which are given a thin layer of metal as an additional coating. Objects are usually electroplated to make them more attractive to look at or to help them resist corrosion. Car, motorcycle and bike parts for example are often electroplated with chromium metal to make them look shiny and also to help them resist corrosion. Engine parts, exhausts, mirrors and bumpers on cars and motorcycles are commonly electroplated. Inexpensive jewellery or costume jewellery is often made of a cheaper less expensive metal which is then silver or gold plated. Rings, bracelets, watches are often electroplated. Cutlery maybe electroplated with silver, an unreactive metal which will resist corrosion from acids found in certain foods. The image below shows common everyday metal objects which are electroplated:


Tin cans which contain many different types of foodstuffs are actually made of the alloy
steel and not the metal tin. Although
most steel cans have a layer of the less reactive metal
tin coating the inside of it; tin being an unreactive metal resists
corrosion and attack by any acids in the foodstuffs better than the steel can.
The cans benefit from the strength of the steel while the electroplated tin cans are manufactured by first forming the steel into the desired can shape. The steel is then cleaned and coated with a thin layer of tin, usually through a process called electroplating. This involves immersing the steel can in a solution containing tin ions and applying an electric current, this causes a thin layer of tin to be deposited onto the steel can, creating a protective layer or tin coat on the can; this makes use of tin's lack of chemical reactivity; though many steel cans are also lined with a thin plastic coating to provide additional protection that prevents any direct contact between the food and the metal, which can help avoid potential chemical reactions and contamination taking place
However if the can is dropped or damaged such that
the protective tin coating is broken then the steel can will start to rapidly
corrode and spoil the food inside the can. A more reactive metal
when connected to a less reactive metal will
"sacrifice" itself to prevent the corrosion of the less
reactive metal. We can take advantage of this
to help slow down the corrosion of metals
e.g.

A more reactive metal can be used to help prevent a less reactive metal from corroding, recall that when a metal corrodes it is oxidised; that is it loses electrons; however if this metal is in contact with a more reactive metal than itself it will not be oxidised and therefore it will not corrode. The more reactive metal will be oxidised instead, it will lose electrons to the less reactive metal and so stop it being oxidised or corroding; the more reactive metal sacrifices itself to protect the less reactive metal, this method of corrosion prevention is called sacrificial protection for obvious reasons.

Cars are zinc coated or galvanised to prevent corrosion, this means that even if the paint is chipped and the steel is exposed to air and water no corrosion will occur. Boats and oil rigs have a similar method of corrosion protection. Boats have large pieces of zinc plate bolted to the underside of their hulls and oil rigs have long zinc strips attached to their legs. These zinc strips help prevent corrosion by sacrificing themselves and corroding to protect the steel which makes up the boats and oil rigs. Periodically these zinc blocks will have to be replaced with fresh blocks as they slowly corrode away.
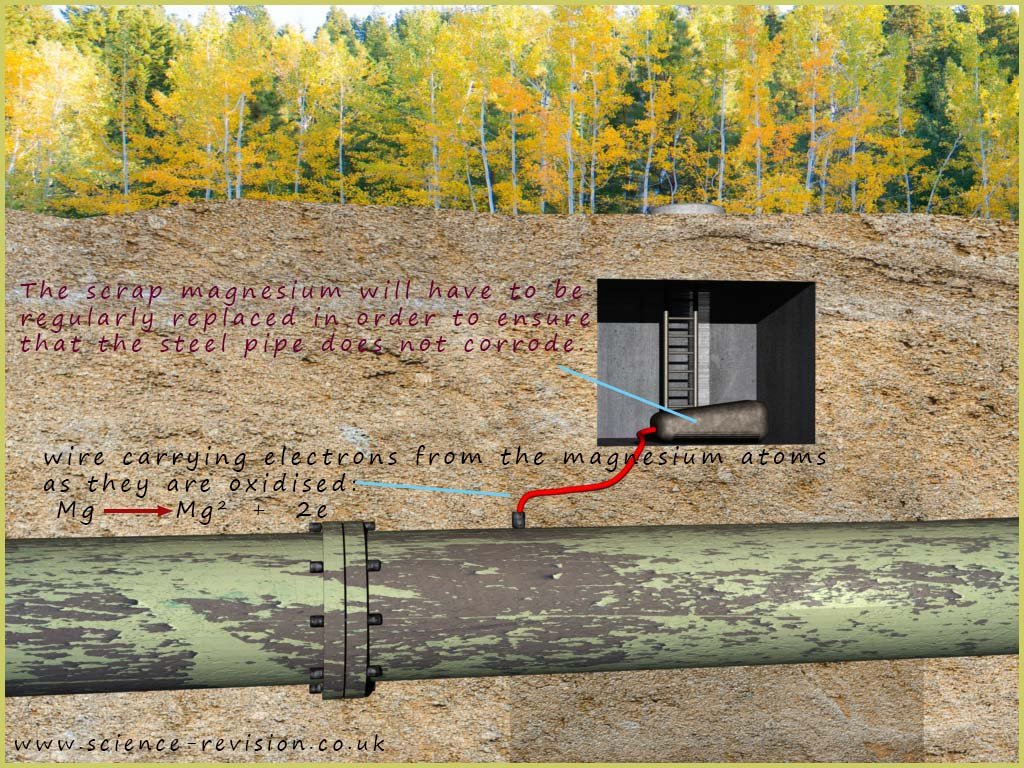
It is not necessary for the object to be completely covered in a more reactive metal in order for it to be protected from corrosion, as was the case with galvanising. As long as the two metals are in contact then corrosion can be prevented. As an example consider underground steel pipes which can be protected from the effects of corrosion by being connected to pieces of scrap magnesium (a more reactive metal) by a length of wire. The magnesium will corrode or be oxidised and send electrons down the wire which will prevent the steel pipes from corroding. This is simply another example of sacrificial protection. It is sometimes referred to as cathodic protection. The image below shows an underground steel pipe being protected from corrosion.
Alloys are mixtures of metals and occasionally non-metals. Mild steel is an alloy made by mixing 99.5% iron with
0.5% carbon. The small amount of carbon makes the iron harder and stronger, mild steel's strength makes it a valuable
material for use in bridge building, construction and in making motor car bodies. However mild steel is liable to undergo
corrosion.
 If iron is mixed with chromium (20%) and nickel (10%) a new alloy called stainless steel is made. Stainless steel is
harder and stronger than mild steel and it does not corrode, but it is very expensive to produce. Brass (70% copper,
30% zinc) and bronze (90% copper, 10% tin) are another two alloys which are
corrosion resistant. They are used to
make items such as statues, monuments and musical instruments.
If iron is mixed with chromium (20%) and nickel (10%) a new alloy called stainless steel is made. Stainless steel is
harder and stronger than mild steel and it does not corrode, but it is very expensive to produce. Brass (70% copper,
30% zinc) and bronze (90% copper, 10% tin) are another two alloys which are
corrosion resistant. They are used to
make items such as statues, monuments and musical instruments.