Higher and foundation tiers
Following the work of early chemists such as Dmitri Mendeleev great strides were made in the development
of a periodic table of elements. With the discovery of new sub-atomic particles such as
electrons and protons
scientists began to understand the fundamental nature of atoms. The addition of an extra column to Mendeleev's periodic
table with the discovery by the Scottish chemist William Ramsay of four Noble gases (neon, argon, krypton and xenon)
and the development of the Rutherford-Bohr model of the atom lead to the
idea that electronic arrangements were important in explaining the chemistry of the
elements and how they react.
 Mendeleev arranged the elements
in his periodic table by their atomic mass but there were some nagging problems that he had
with some elements
in his periodic table; such as iodine and tellurium where he had to swap their positions in his periodic table
and break his own rule of arranging the elements
by their atomic masses. However it was not until well after
Mendeleev's death in 1907 following the work of the brilliant physicist Henry Moseley that an explanation for this problem was provided. Moseley bombarded certain
elements with "cathode rays" (high energy electrons)
and by analysing the frequencies
of the x-rays emitted
realised that the atomic number of an element was due to the number of
positive charges in the nucleus. This led to a reorganisation of the periodic table with
the elements now being arranged by their atomic number and not their masses.
Mendeleev arranged the elements
in his periodic table by their atomic mass but there were some nagging problems that he had
with some elements
in his periodic table; such as iodine and tellurium where he had to swap their positions in his periodic table
and break his own rule of arranging the elements
by their atomic masses. However it was not until well after
Mendeleev's death in 1907 following the work of the brilliant physicist Henry Moseley that an explanation for this problem was provided. Moseley bombarded certain
elements with "cathode rays" (high energy electrons)
and by analysing the frequencies
of the x-rays emitted
realised that the atomic number of an element was due to the number of
positive charges in the nucleus. This led to a reorganisation of the periodic table with
the elements now being arranged by their atomic number and not their masses.
Following the discovery of isotopes it became clear
as to why Mendeleev had to swap
around some elements in his periodic table; particularly the problem
Mendeleev had with masses of the elements iodine and tellurium. If
Mendeleev arranged these two elements according to their
atomic masses then iodine and tellurium would have to swap places
in the periodic table. Mendeleev
swapped these two elements around in his table based on their
chemical properties but he had
no idea why they did not fit his pattern other than that the atomic masses
may have been
calculated wrongly.
Though it made sense to put iodine in group 7; the halogens; since
its chemical reactions were similar to the other halogens. However iodine has a mass of 127 and
tellerium 128. Following the discovery of isotopes it was
found that tellurium has several
isotopes with high atomic masses
and high percentage abundances which means its average atomic mass is
higher than that of iodine.
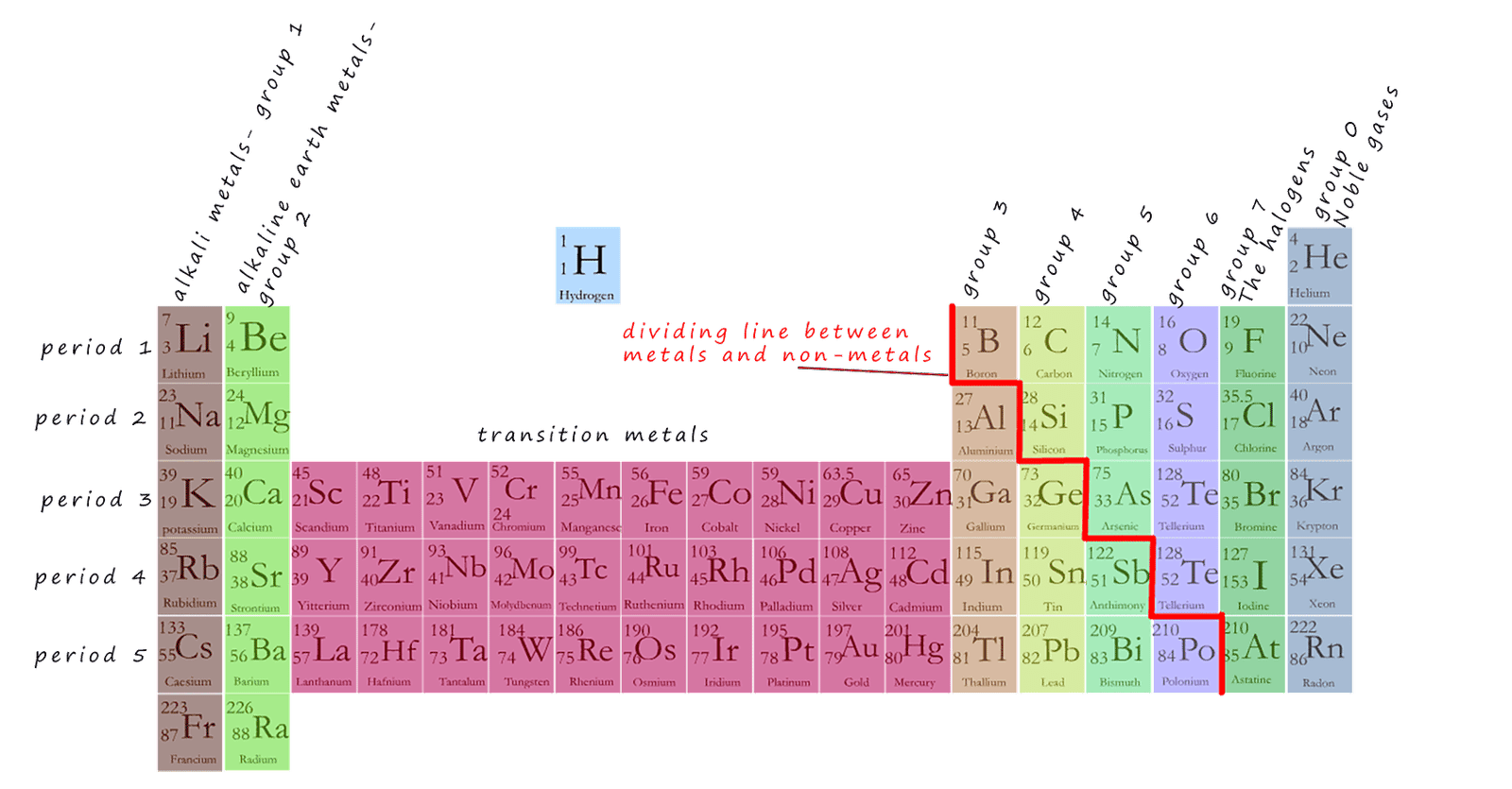 The periodic table makes it easy to predict and explain many of the properties of the elements. You
should be aware of the following facts:
The periodic table makes it easy to predict and explain many of the properties of the elements. You
should be aware of the following facts: