
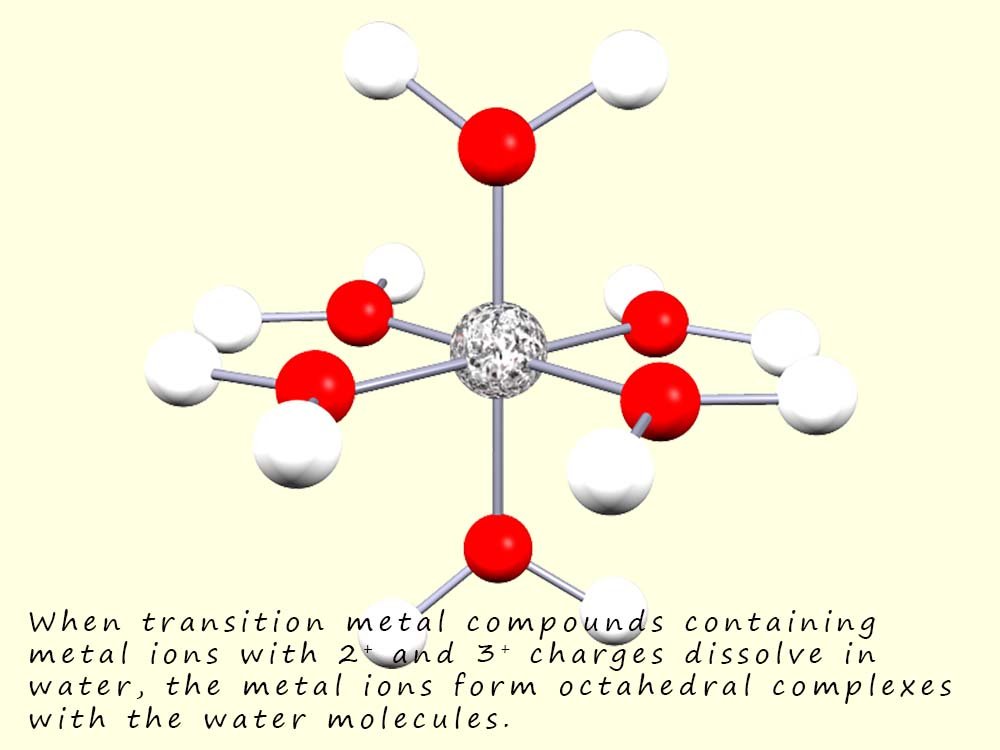
When solid transition metal compounds containing a metal ion in the 2+ or 3+ oxidation states are dissolved in water, the water molecules can use one of their lone pairs of electrons to act as a Lewis base and a form coordinate bond with the metal ion, this results in the formation of complex ions which typically consist of octahedral complexes; as shown in the image opposite.
Many of the solutions containing these hexaaqua ions have vivid and striking colours, for example:
The colours of these three aqueous solutions are shown in the image opposite.
The reaction in which hydrated ions, such as hexaaqua ions are formed involves the dissolution (this is simply the process when a solute dissolves in a solvent to form a solution) of one mole of gaseous ions in water, and the associated enthalpy change is called the enthalpy of hydration. This enthalpy change contributes to overcoming the lattice enthalpy—the energy required to break apart the ionic lattice when a substance dissolves.
Generally, if the enthalpy of hydration is larger than the lattice enthalpy, then the enthalpy of solution will be exothermic and more than likely it will be a thermodynamically favourable process. However, entropy changes can also play a significant role in determining the overall feasibility of dissolution or solution formation.
water, and the associated enthalpy change is called the enthalpy of hydration. This enthalpy change contributes to overcoming the lattice enthalpy—the energy required to break apart the ionic lattice when a substance dissolves.
Generally, if the enthalpy of hydration is larger than the lattice enthalpy, then the enthalpy of solution will be exothermic and more than likely it will be a thermodynamically favourable process. However, entropy changes can also play a significant role in determining the overall feasibility of dissolution or solution formation.
The enthalpy of hydration and lattice enthalpy are connected through the enthalpy change of solution, as described by the following equation:
Where the lattice enthalpy (ΔHlattice) is:
The energy required to break one mole of a solid ionic lattice into its separate gaseous ions, this process will be obviously be an endothermic one since it involves bond breaking, that is it will require an input of energy,
for example the lattice enthalpy or lattice dissociation enthalpy of the ionic compound sodium chloride (NaCl) can be shown by the equation:

So we can say that if:
This equation above helps to explain why some ionic compounds dissolve easily in water, while others do not. The balance between the energy required to break down the ionic lattice and the energy released during hydration helps us in determining the solubility. If ΔHsolution is negative, that is dissolution is exothermic then it will likely be thermodynamically favourable, although the substance may still be soluble if ΔHsolution has a positive enthalpy value as long as there is an increase in entropy during the process which leads to a negative value for the Gibbs free energy change for solution e.g.
If the Gibbs free energy of solution is given by the equation:
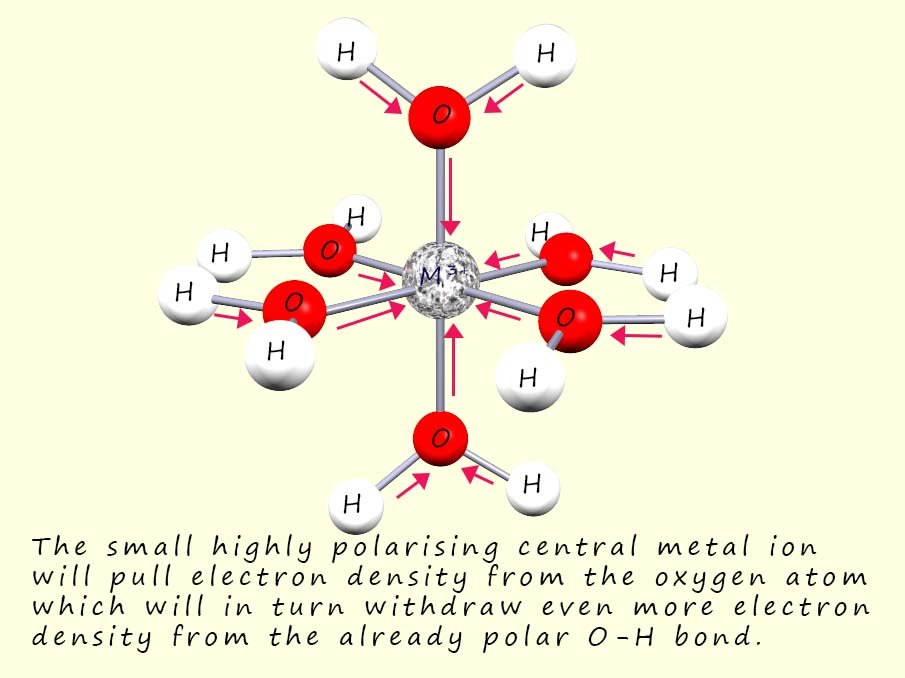 When a transition metal ion forms a complex ion the small highly charged central metal ion will polarise or distort the electron density in the water molecules bonded to it, that is it will pull or withdraw electron density from the water ligands. The M-O bond will become polarised with the metal ion withdrawing electron density from the oxygen atom present in the water molecule. The larger the size of the charge on the central metal ion and the smaller its radius then the more it will be able to polarise and withdraw electron density towards it, this will weaken the O-H bond in the water ligand and make it easier for the loss of a hydrogen ion (H+) to occur, this is outlined in the image opposite; where the red arrows indicate the direction of electron flow towards the central metal ion, in this case a metal ion with an oxidation state of 3+.
When a transition metal ion forms a complex ion the small highly charged central metal ion will polarise or distort the electron density in the water molecules bonded to it, that is it will pull or withdraw electron density from the water ligands. The M-O bond will become polarised with the metal ion withdrawing electron density from the oxygen atom present in the water molecule. The larger the size of the charge on the central metal ion and the smaller its radius then the more it will be able to polarise and withdraw electron density towards it, this will weaken the O-H bond in the water ligand and make it easier for the loss of a hydrogen ion (H+) to occur, this is outlined in the image opposite; where the red arrows indicate the direction of electron flow towards the central metal ion, in this case a metal ion with an oxidation state of 3+.
The withdrawal of electron density from the already polar O-H bond in the water ligand allows for the loss of a hydrogen ion (H+), which obviously results in the formation of an acidic solution. Now it is important to realise that the hydrogen ion (H+) does not simply just "fall off" the water ligand, instead another water molecule will act as a Brønsted-Lowry base and remove one of the hydrogen ions (H+) to form a hydronium or hydroxonium ion (H3O+) We can represent this as using the equation below, here the complex ion contains a metal ion with a 3+ oxidation state:
The H3O+ ion is called the hydronium or hydroxonium ion and it is usually simply represented as H+ in many chemical equations, you can simplify the equation above to give:
However you should bear in mind that the hydrogen ion (H+) in the above equation is actually a hydronium ion (H30+) that forms when a water molecule acts as a base and removes a hydrogen ion (H+) from the [M(H2O)6]3+ complex as outlined in the image below:
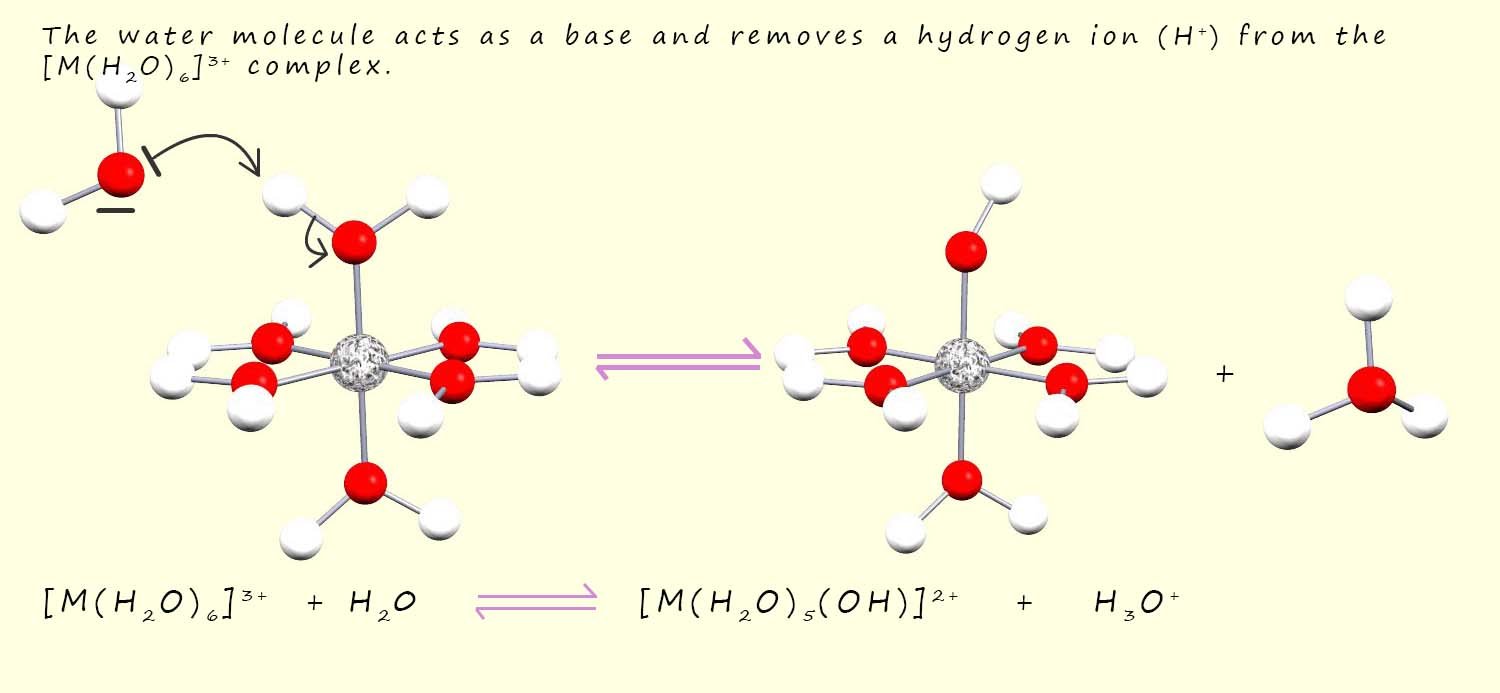
In this equilibrium reaction a water molecule bonded to the metal ion has been split or broken up into a hydrogen ion (H+) ion and a hydroxide ion (OH-) ion so for this reason the reaction is often referred to as a hydrolysis reaction but it is also commonly referred to as an acidity reaction because a hydronium ion (H3O+) is formed.
Now there are obviously six water ligands surrounding the central metal ion in a hexaaqua complex and so far we have only considered the loss of one hydrogen ion (H+) from one of the water ligands, however you can get the loss of additional hydrogen ions (H+) from the remaining water ligands:
So far we have seen three equations; shown below to show what forms when a metal ion in the 3+ oxidation state is dissolved in water and it can lose up to three hydrogen ions (H+) before a solid precipitate forms, now all of these species will be present in the equilibrium mixture formed but the concentration of each species present will depend on the concentration of the solution, with the [M(H2O)6]3+(aq) ion being the main ion present in the equilibrium mixture in dilute solutions, though the concentration of the other ions present will increase as the concentration of the complex increases or the pH is raised by the additional of a base. If the concentration is sufficiently high then a solid precipitate may be seen.
Hexaaqua complexes which contain metal ions with a 2+ oxidation state will only lose two hydrogen ions (H+) before a neutral precipitate forms, the two equations below are very similar to the ones above and show the loss of two hydrogen ions (H+) from an hexaaqua metal complex:
When hexaaqueous complexes undergo chemical reactions then there are really only two possibilities that can take place:
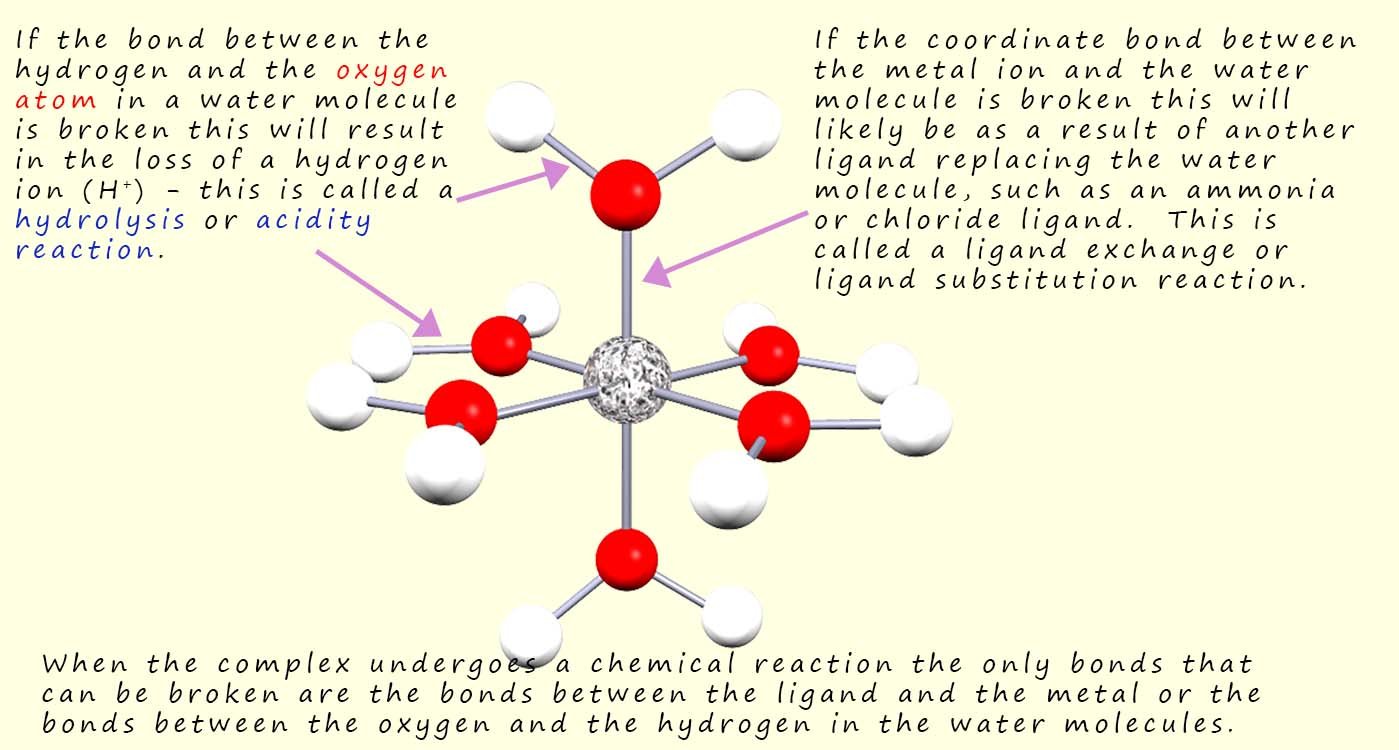
For the moment let us consider the first hydrolysis reaction only that takes place in a hexaaqueous complex containing metal ions with oxidation states of 2+ and 3+, now we can represent the equilibrium reaction that takes in the solutions containing these complex ions as:

 Complexes which contain the metal (II) ions have typical values for the acid dissociation constant Ka of between 10-6 and 10-11 while complexes containing the metal (III)
ions the value of Ka varies between 10-2 and 10-5. These very small values of Ka clearly indicate that the main species present in these two equilibrium reaction are the hexaaqua ions and that the position of equilibrium lies very much to the left hand side of the two equations above.
These figures do however show that for complexes containing the metal (II) ions about 1 in 10, 000 of the hexaaqua ions undergoes hydrolysis while for the metal (III) complexes about 1 in 1000 undergoes hydrolysis, or we can say that solutions of metal (II) ions will have a pH of around 6 while solutions of complexes containing the metal (III) ions will have a pH of around 3. That is solutions containing metal (III) ions are about 1000 times more acidic than solutions containing a metal (II) ion.
Complexes which contain the metal (II) ions have typical values for the acid dissociation constant Ka of between 10-6 and 10-11 while complexes containing the metal (III)
ions the value of Ka varies between 10-2 and 10-5. These very small values of Ka clearly indicate that the main species present in these two equilibrium reaction are the hexaaqua ions and that the position of equilibrium lies very much to the left hand side of the two equations above.
These figures do however show that for complexes containing the metal (II) ions about 1 in 10, 000 of the hexaaqua ions undergoes hydrolysis while for the metal (III) complexes about 1 in 1000 undergoes hydrolysis, or we can say that solutions of metal (II) ions will have a pH of around 6 while solutions of complexes containing the metal (III) ions will have a pH of around 3. That is solutions containing metal (III) ions are about 1000 times more acidic than solutions containing a metal (II) ion.