
A complex is a compound which usually consists of a central transition metal ion or atom which is surrounded by a number of atoms, molecules or ions that are bonded to the central transition metal atom/ion by coordinate or dative covalent bonds. The image below shows a complex formed between a transition metal ion and six water molecules. In this complex the water molecules use one of their lone pairs of electrons to form a dative or coordinate covalent bond to the transition metal ion. That is the metal ion is acting as a lone pair acceptor or a Lewis acid while the water molecules are acting as a Lewis base or an electron pair donor.
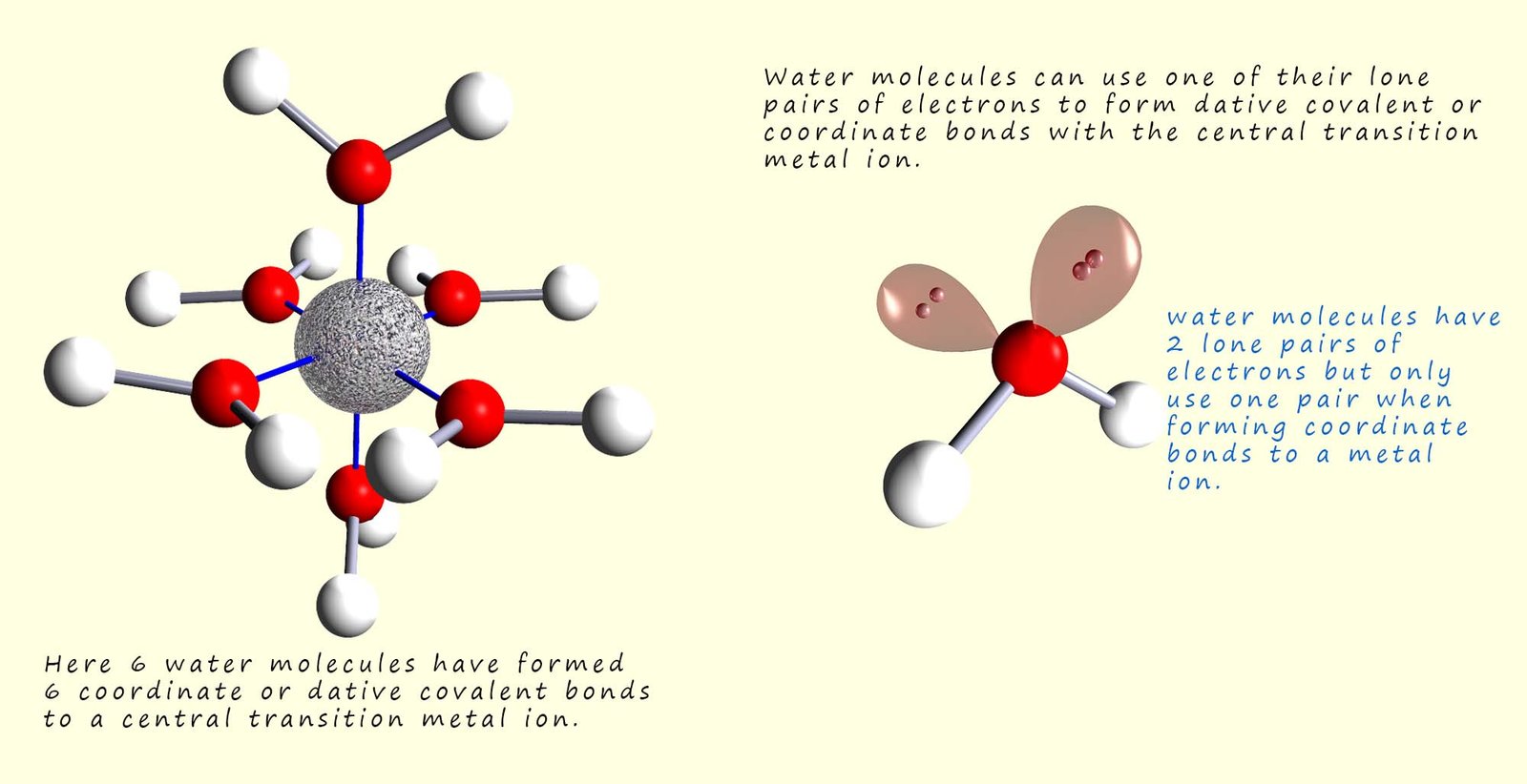
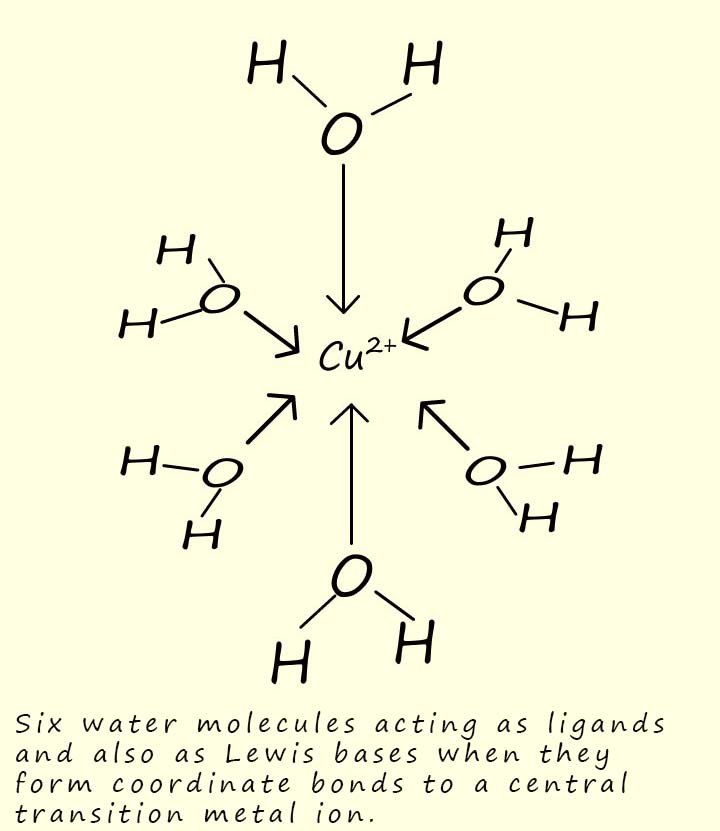 The molecules and ions that bond to the central transition metal ion in these complexes are called ligands (from the Latin word ligare; which means to bind). These ligands are species that are able to donate a pair of electrons into empty orbitals in the central transition metal ion, that is the ligands are Lewis bases or electron pair donors while the central transition metal ion is a Lewis acid or an electron pair acceptor. The coordinate bonds formed by the ligands to the central metal atom/ion are often shown using arrows, as outlined in the diagram opposite. Common ligands that you are likely to meet include:
The molecules and ions that bond to the central transition metal ion in these complexes are called ligands (from the Latin word ligare; which means to bind). These ligands are species that are able to donate a pair of electrons into empty orbitals in the central transition metal ion, that is the ligands are Lewis bases or electron pair donors while the central transition metal ion is a Lewis acid or an electron pair acceptor. The coordinate bonds formed by the ligands to the central metal atom/ion are often shown using arrows, as outlined in the diagram opposite. Common ligands that you are likely to meet include:
As a simple example consider the reaction of ammonia (NH3) with silver ions (Ag+), here the complex formed has a linear shape. Two ammonia (NH3) molecules are able to act as Lewis bases and form coordinate bonds with vacant orbitals on the silver ion, equations for this Lewis acid-Lewis base reaction are shown below:
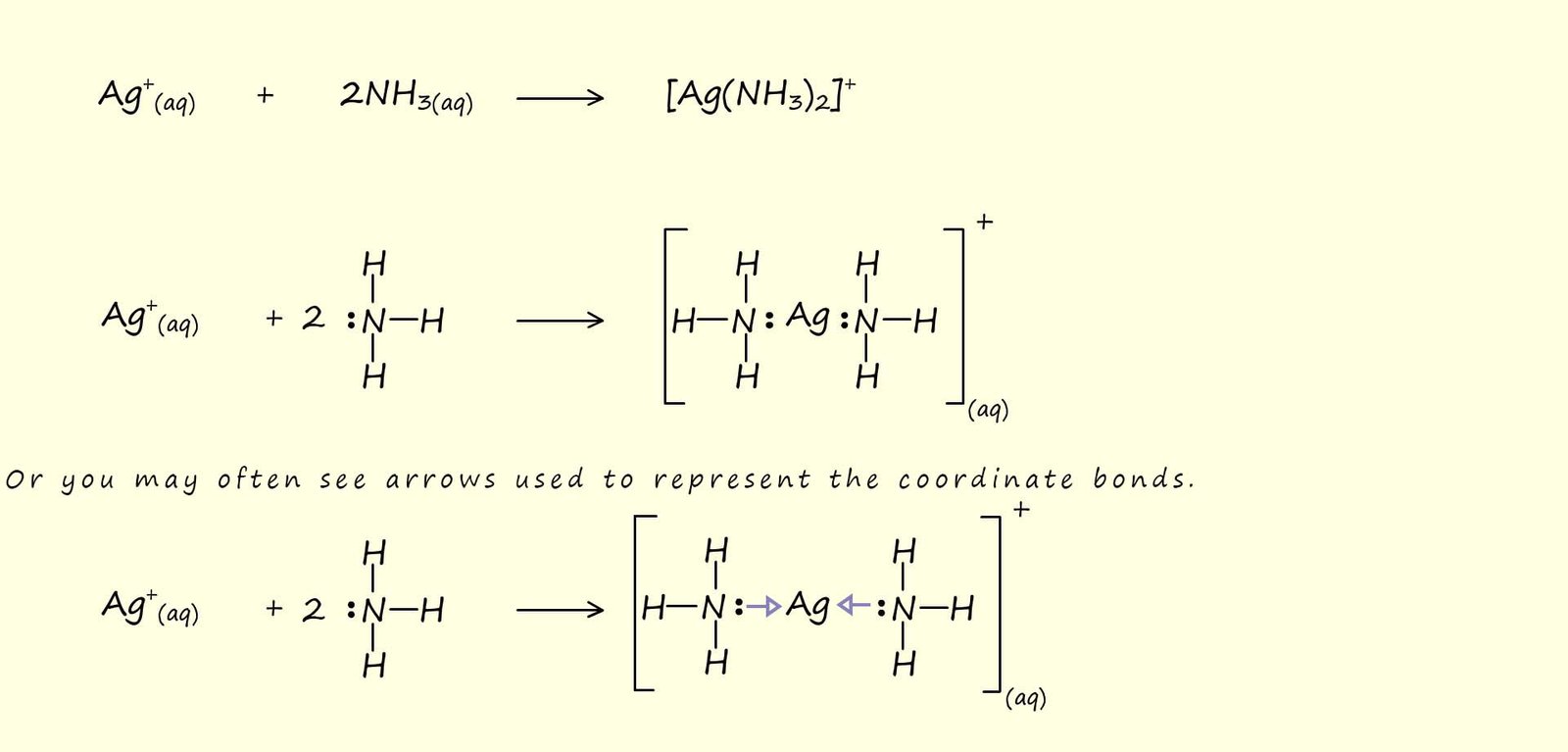
In this simple example the complex is shown in square brackets [ ] while the ammonia ligands are shown using in parenthesis ( ), the overall charge on the complex is show as a superscript outside the square brackets.
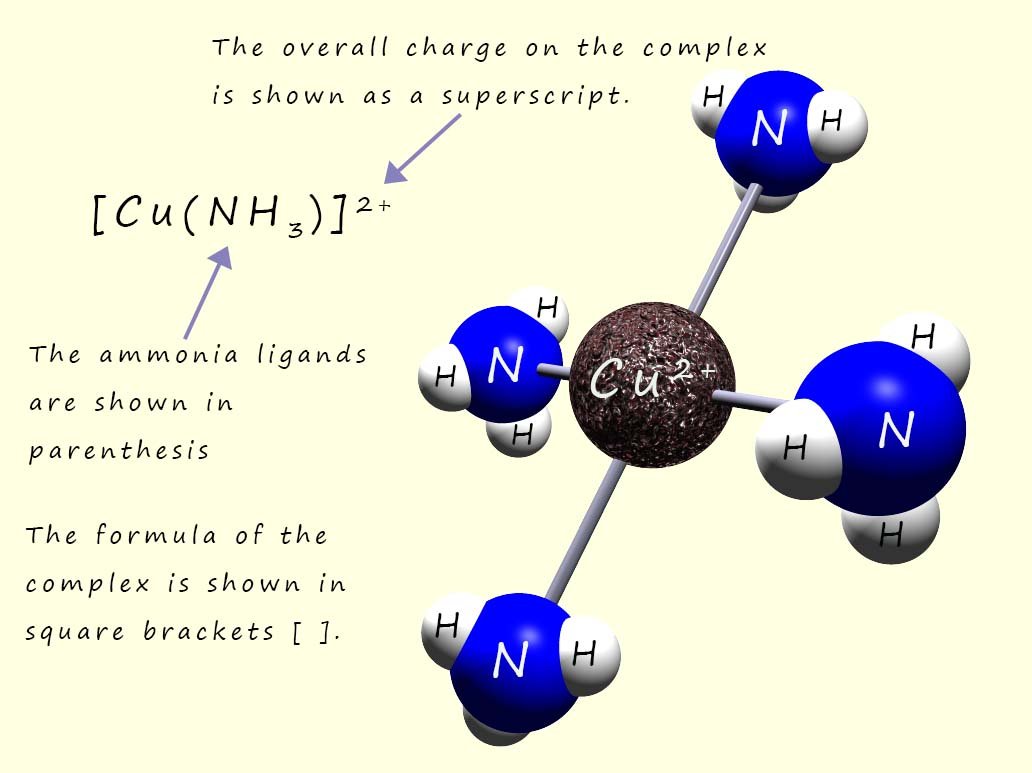 As a second example consider the square planar complex formed between copper ions (Cu2+) and four ammonia ligands (shown in the image opposite).
In this example the formula of the complex is written as [Cu(NH3)4]2+, the ammonia ligands (NH3) are neutral molecules and so have no charge; this means that the 2+ charge on the complex is due only to the charge on the copper ion (Cu2+).
As a second example consider the square planar complex formed between copper ions (Cu2+) and four ammonia ligands (shown in the image opposite).
In this example the formula of the complex is written as [Cu(NH3)4]2+, the ammonia ligands (NH3) are neutral molecules and so have no charge; this means that the 2+ charge on the complex is due only to the charge on the copper ion (Cu2+).
Example 3- The [Cr(H2O)4Cl2]+ complex is shown in the image below:
![3D model [Cr(H20)4Cl2)]+ complex ion](images/complex with water and chloride.jpg)
In the [Cr(H2O)4Cl2]+ complex there are two types of ligands, four neutral water molecules and two negatively charged chloride ions (Cl-); overall this complex has a charge of +1; this means that the chromium ion must have a charge of +3 since there will be a total charge of -2 from the two chloride ion (Cl-) and the presence of a Cr3+ ion will give the complex an overall charge of +1.
Many of these complexes have an overall charge and are therefore likely to exit as ionic compounds where a counter ion or ions are present such as a sodium ions (Na+) or potassium ions (K+), these ionic salts are often referred to as coordination compounds though you will often see the words coordination compound and complex used interchangeably, for example
4.jpg)
You should be aware of the fact that within these salts the bonding between the complex and the counter ions present may be ionic but the bonding within the complex itself is coordinate ; that is dative covalent bonds exist between the metal ion and the ligands. Therefore, these coordination compounds exhibit a combination of both ionic and covalent bonding, contributing to their unique properties and structures.
However not all complexes are ionic; many are not; for example the tetracarbonylnickel Ni(CO)4 complex (shown in the image opposite) is neutral with no ionic components. The nickel atom is covalently bonded to four carbon monoxide or carbonyl ligands (CO).
The number of ligands that surround the central metal ion or atom in a coordination compound or a complex is called the coordination number. The coordination number of a metal ion in a complex depends on a number of factors; which include:
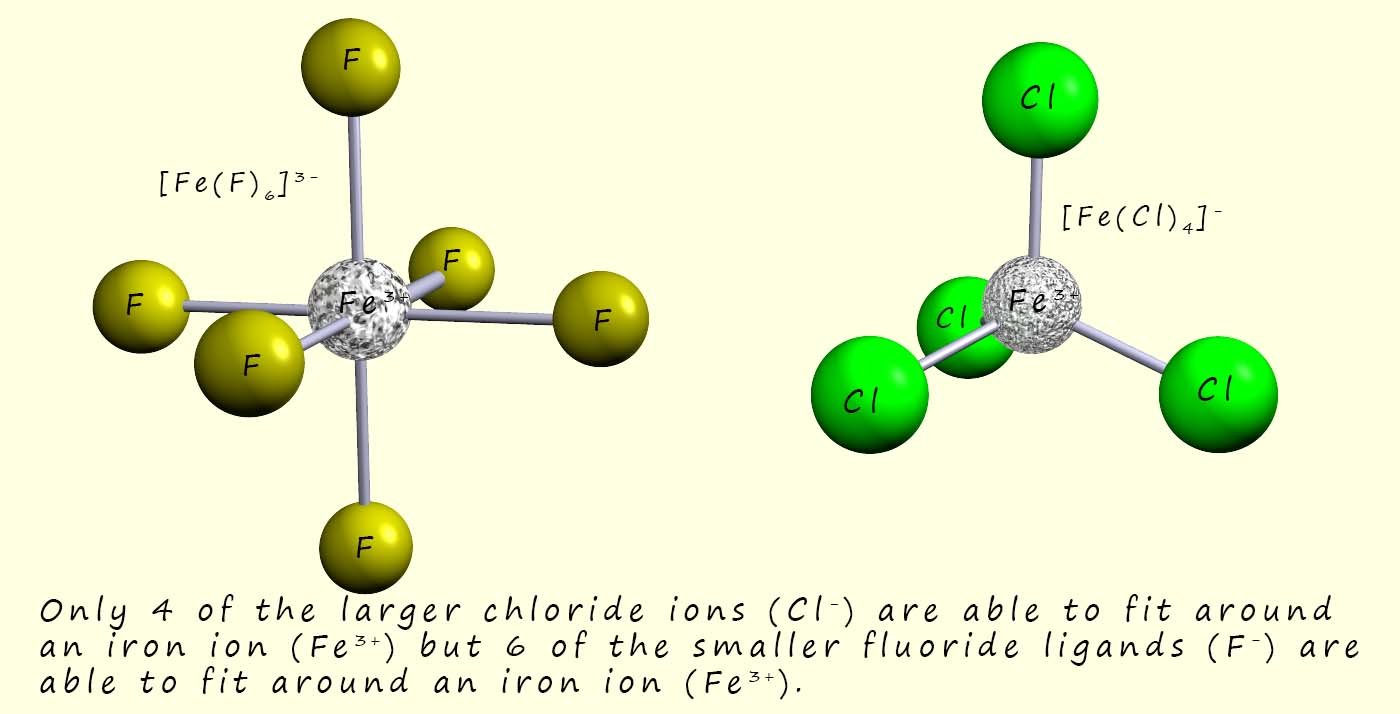
The most common shapes for the complexes formed by the first row of transition metals in the periodic table are shown in the image below.
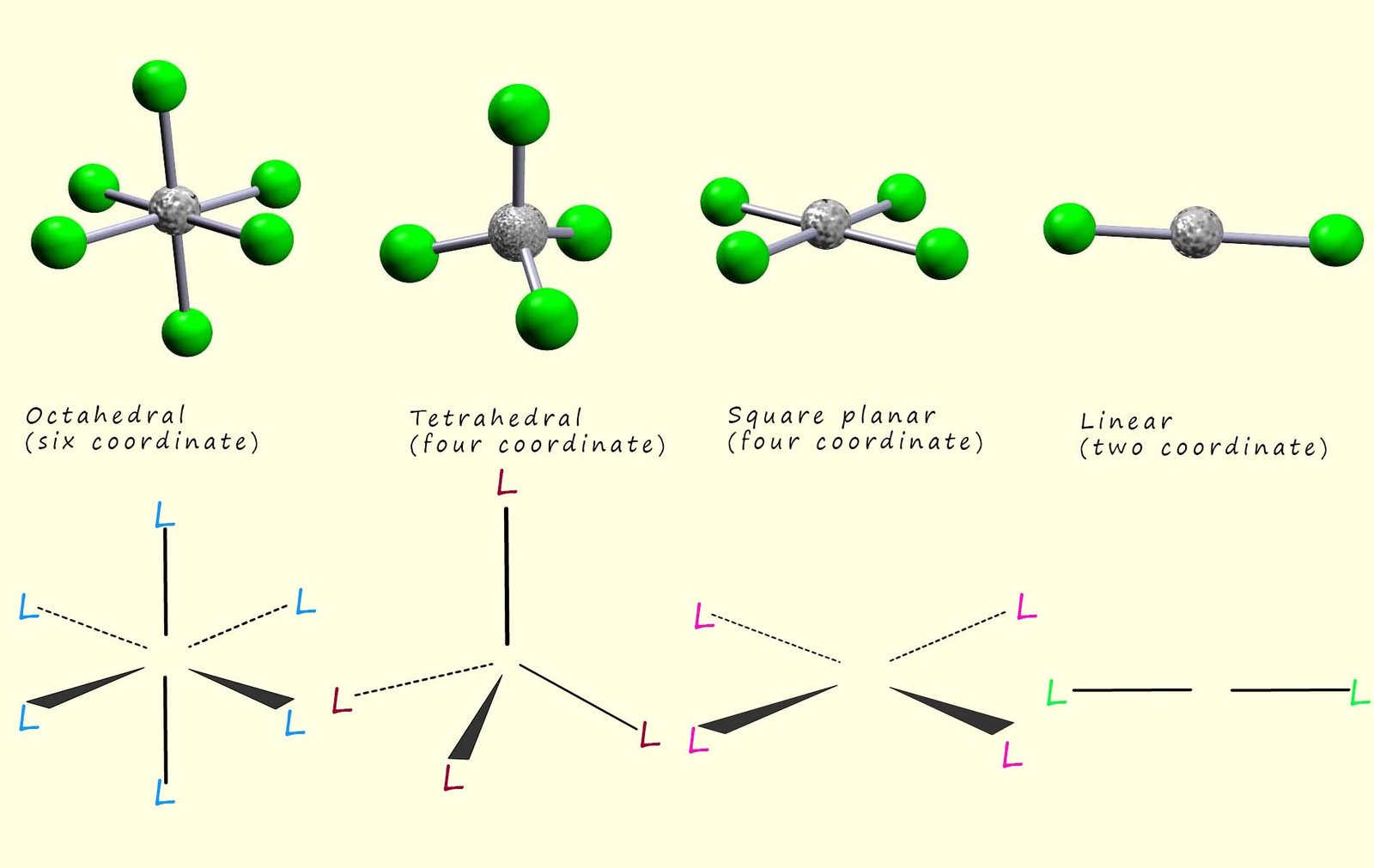
The octahedral geometry is the most common amongst complexes which are six coordinate, while four coordinate complexes are either tetrahedral or square planar; the tetrahedral arrangement is much more common than the square planar geometry tough the square planar geometry is often found in transition metal ions with 8d electrons in the valence shell; for example in platinum (II) and copper(II) ions. Silver (I), Copper (I), Gold (I) and other ions with a d10 electronic configuration ions frequently form linear or two coordinate complexes; for example the following coordination compounds all have a linear geometry: