One way in which the transition metals differ from the metals in groups I, II and III in the periodic table is the fact that can have a variety of different oxidation states. While a group II metal such as magnesium only ever has an oxidation state of 2+ and an alkali metal in group I in the periodic table will mostly form compounds where the oxidation state is +1, the transition metals form a variety of compounds where the metal can have a number of different oxidation states.
One way in which the transition metals differ from the metals in groups I, II and III in the periodic table is the fact that can have a variety of different oxidation states. While a group II metal such as magnesium only ever has an oxidation state of 2+ and an alkali metal in group I in the periodic table will mostly form compounds where the oxidation state is +1, the transition metals form a variety of compounds where the metal can have a number of different oxidation states.
All the first row transition metals for example except scandium form ions with a 2+ charge; corresponding to the loss of the 4s valence electrons (Sc forms a particularly stable ion with a 3+ charge; Sc3+).
The 3d sub-level in a transition metal is very close in energy to the 4s sub-level so the electrons present in the 3d sub-level are also relatively easily lost to form ions with 3+ charges; for example consider the two metals vanadium and chromium both of which will form ions with a 3+ charge:
The common oxidation states for the first row transition metals are shown in the table below, although the particular oxidation state of the metal will depend on the reaction conditions and the reagents used in any one particular reaction.
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| +1 | ||||||||
| +2 | +2 | +2 | +2 | +2 | +2 | +2 | +2 | |
| +3 | +3 | +3 | +3 | +3 | +3 | +3 | ||
| +4 | +4 | +4 | ||||||
| +5 | ||||||||
| +6 | +6 | |||||||
| +7 |
The transition metal ions with higher oxidation states such as +6 and +7 are found in complexes where the transition metal is bonded to very electronegative elements such as oxygen and fluorine, for example the chromate ion (CrO42-) contains chromium atoms with an oxidation state of +6 while the manganate ion (MnO4-) contains manganese atoms with an oxidation state of +7
I am sure you will have seen the names of many transition metals written with the oxidation number of the metal present in the compound written in Roman numerals e.g.
The lower oxidation states of the transition metals mostly consist of simple ions such as M2+ and M3+. Transition metals with a low oxidation number can act as reducing agents and donate electrons to other substances e.g. in the two equations below the transition metal ions act as reducing agents and lose an electron and increase their oxidation state by one.
As previously mentioned the higher oxidation states for the transition metals are only found in compounds where the metal is bonded to an element with a high electronegativity value, such as oxygen and fluorine e.g.
Ions which contain metals in high oxidation states are good electron acceptors; that is they are good oxidising agents.
The transition metal vanadium has a wide range of oxidation states ranging from +2, +3, +4 and +5, now each of these
oxidation states
has a different colour. You can view all the different colours of these various oxidation states of vanadium by simply reducing vanadium ions in the +5 oxidation state using zinc metal in acid as the reducing agent:


The zinc metal is simply acting as the reducing agent here and it supplies the necessary electrons to reduce the dioxovanadium ions according to the equation below:
Further reduction using more zinc will reduce the
green V3+( ions to form the violet V2+(aq) ion.

These reactions can simply be carried out in a conical flask which has a loose plug of mineral or cotton wool in the mouth of the flask, this will allow the hydrogen gas produced in the side reaction between the acid present and the zinc metal; it will also help reduce the influx of air back into the flask since the V2+ and V3+ are readily oxidised by oxygen present in the air.
The vanadium ions mentioned above are all in aqueous solution and they will likely form octahedral complexes due to coordination with water molecules and other ions present such as chloride ions (Cl-). The central vanadium ion in the complex will be surrounded by six ligands, typically water, resulting in an octahedral geometry, for example:
The presence of the water, oxo and chloride ligands is almost always emitted from equations involving the reduction and oxidation of vanadium ions to keep "things" simple.

Chromium is a very useful transition metal; its common properties include:
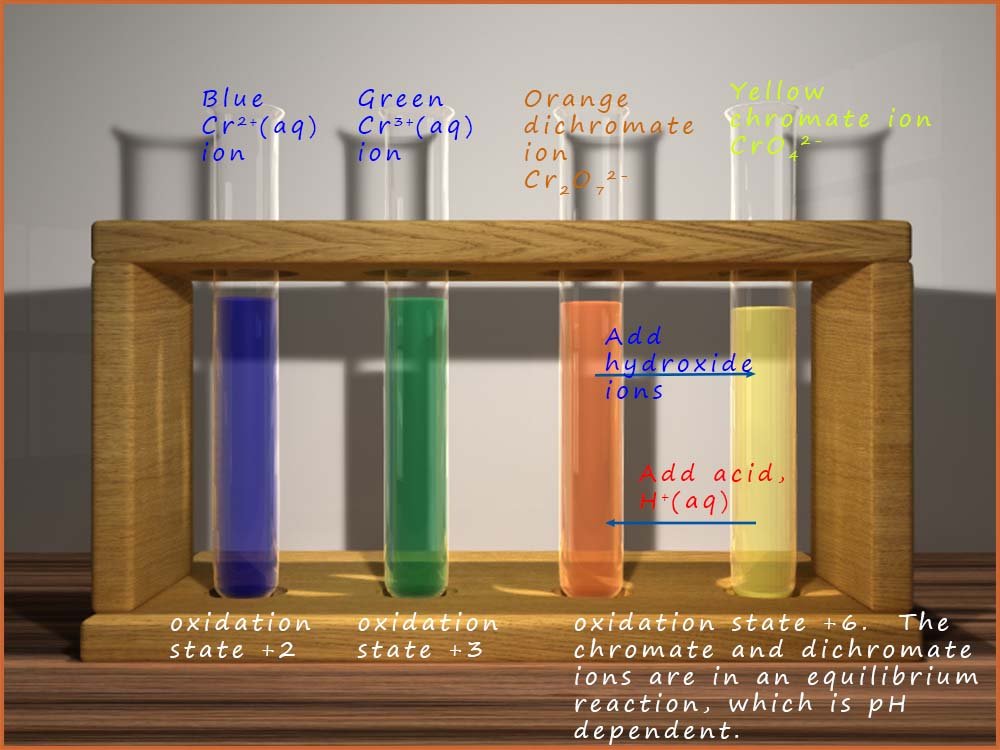
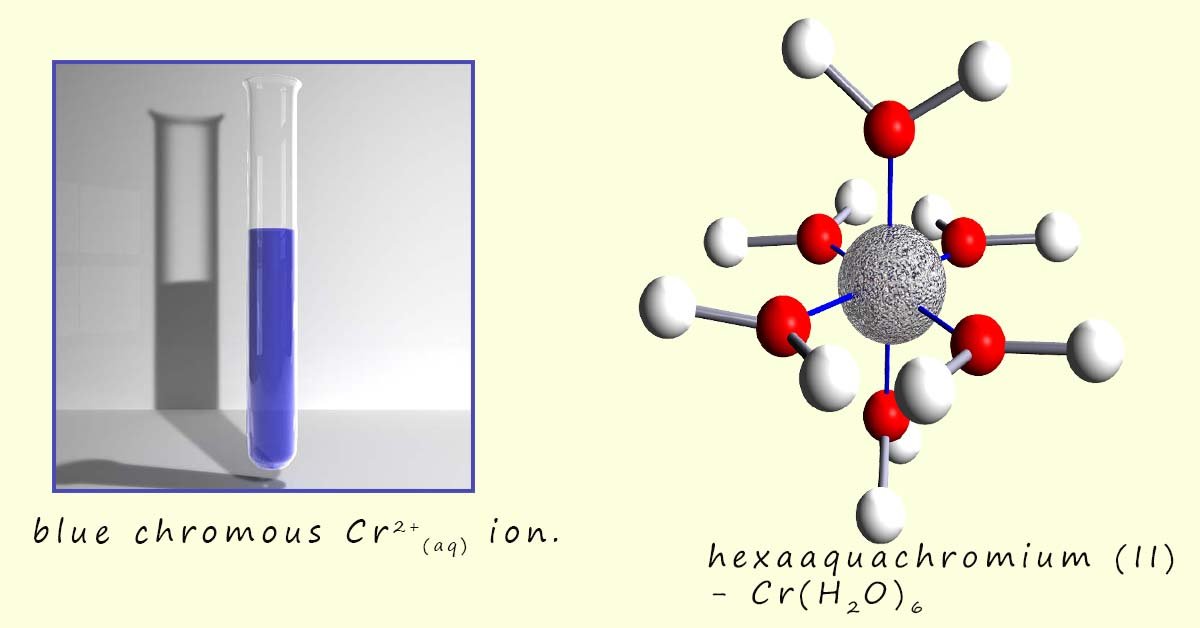
Chromium metal is another transition metal with variable oxidation states, it has 3 common oxidation states, these are: +2, +3 and +6; that is Cr2+, Cr3+ and Cr6+; with the Cr3+ ion being the most stable. Now chromium metal for example will react with concentrated acid in the absence of air to yield the blue chromous ion (Cr2+). Here the Cr2+ ion forms six coordinate bonds with water molecules to form the octahedral complex [Cr(H2O6)]2+; which is shown in the image below. We can write a simplified equation for this reaction as:
The image below shows the blue Cr2+(chromous) ion and the structure of the hexaaquachromium(II) complex which is formed in acidic conditions.
 Cr2+(aq) ions are good a reducing agent and if the above reaction is carried out in the presence of air then
the blue Cr2+(aq) ions are rapidly oxidised by the oxygen in the air to form the green Chromium (III) ion. Although the [Cr(H20)6]3+ ion is actually violet in colour solutions of chromium (III) are usually green in colour due to the presence of anions such as Cl- in the solution which replace one or more of the water molecules in the [Cr(H2O)6]3+ complex to form complex anions such as [Cr(H2O)5Cl]2+ and [Cr(H2O)4Cl2]+ which have a green colour.
Cr2+(aq) ions are good a reducing agent and if the above reaction is carried out in the presence of air then
the blue Cr2+(aq) ions are rapidly oxidised by the oxygen in the air to form the green Chromium (III) ion. Although the [Cr(H20)6]3+ ion is actually violet in colour solutions of chromium (III) are usually green in colour due to the presence of anions such as Cl- in the solution which replace one or more of the water molecules in the [Cr(H2O)6]3+ complex to form complex anions such as [Cr(H2O)5Cl]2+ and [Cr(H2O)4Cl2]+ which have a green colour.
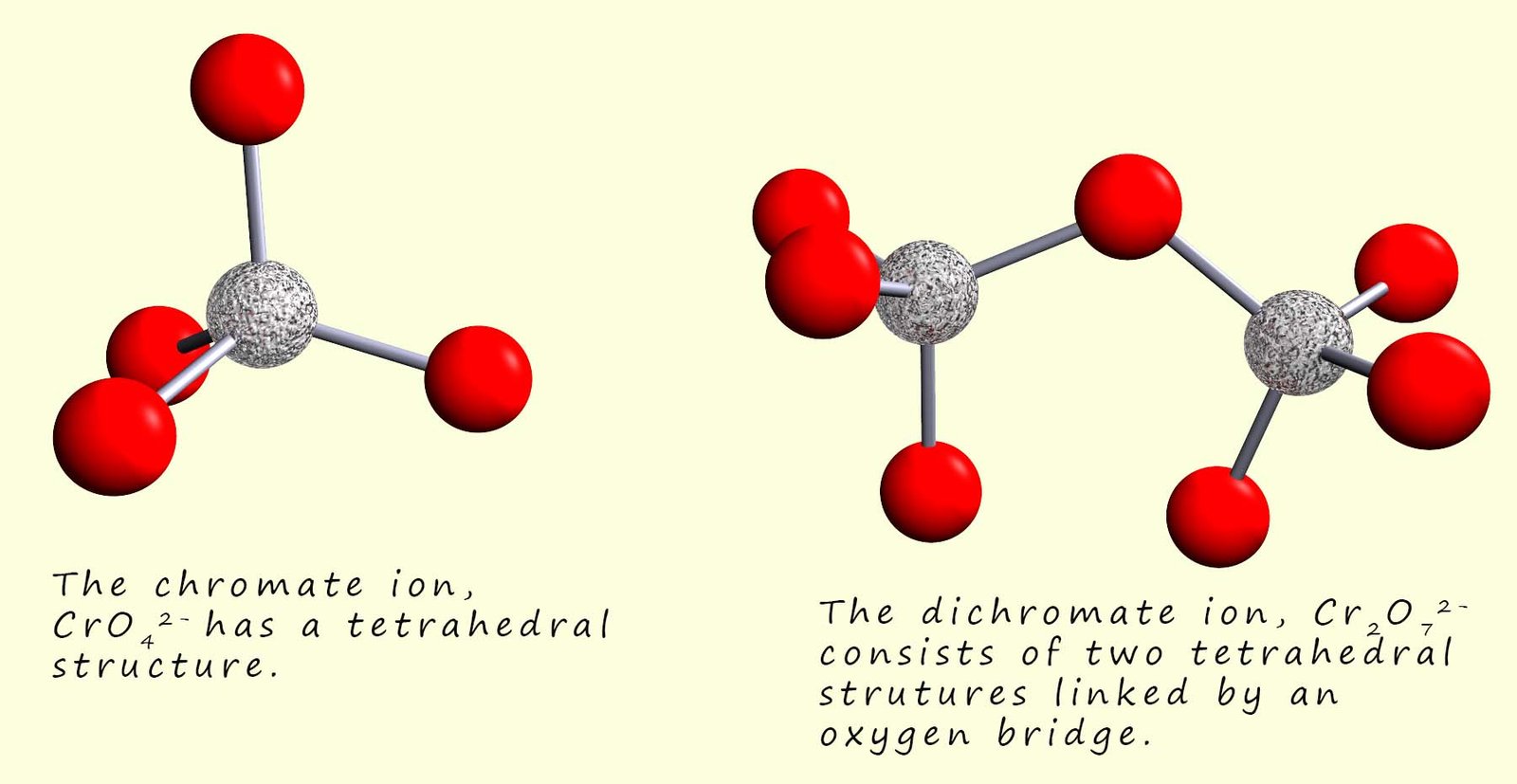
The highest oxidation state for chromium metal is +6 and this is found in two ions, the yellow chromate ion (CrO42-) and the orange dichromate ion (Cr2O72-) ion. The chromate ion (CrO42-) has a tetrahedral structure while the dichromate ion orange dichromate ion (Cr2O72-) consists of two tetrahedral structures which are linked by an oxygen bridge; this is shown in the image below:
These two ions are in equilibrium with each other and the concentration of each ion in the equilibrium mixture depends upon a number of factors including the pH of the solution, the equilibrium reaction between these two species can be shown by the equation below: