The use of the drug cisplatin as an anticancer drug was discovered by accident in 1965 by Dr. Barnett Rosenberg and his colleagues, Loretta VanCamp and Thomas Krigas, at Michigan State University in the USA. Rosenberg was investigating the effects of electric fields on bacterial growth using Escherichia coli (E. coli) bacteria. Platinum electrodes were used to pass the electric current through these bacterial cells since the team believed that platinum; being an unreactive metal would not interfere with the results of their experiments.
Rosenberg observed during his investigations that bacterial cell division was almost completely inhibited; which was a very surprising result indeed. Eventually after much research the cause of this inhibition of bacterial division was traced not to the electric fields used in his experiments but to a platinum containing compound formed by the electrodes during their experiments. This platinum compound was found to contain ammonia and chloride ligands.
Two possible compounds which could be inhibiting the bacterial cell division were identified as [Pt(NH3)2Cl2] and [Pt(NH3)2Cl4]. However further research proved that the active compound was in fact the diamminedichloroplatinum(II)-[Pt(NH3)2Cl2] complex. Now this complex can exist as two geometric isomers, a cis and a trans isomer, the structure of these two isomers is shown in the image below: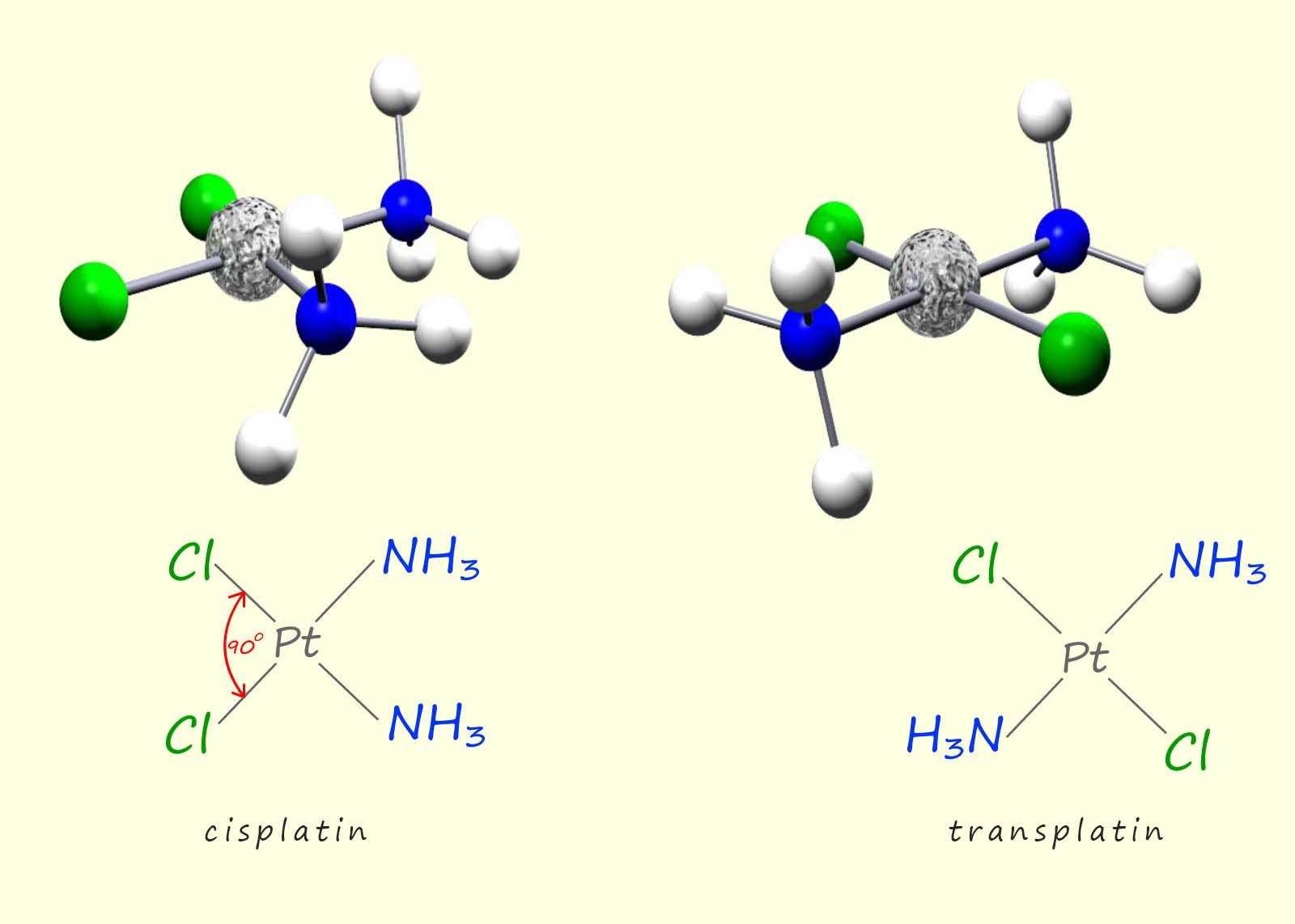
After further investigation it was found that the trans isomer of diamminedichloroplatinum(II) was ineffective at stopping or reducing cell division and the actual active compound was the cis isomer of the diamminedichloroplatinum(II) complex, which was later renamed cisplatin. This square planar complex consists of a platinum ion (Pt2+) with two ammonia (NH3) ligands and two chloride (Cl-) ligands in a cis configuration. The "cis" prefix indicates that the ammonia and chloride ligands are positioned next to each other with the platinum ion at the centre of the complex, this cis arrangement is essential for its anticancer and biological activity.
