
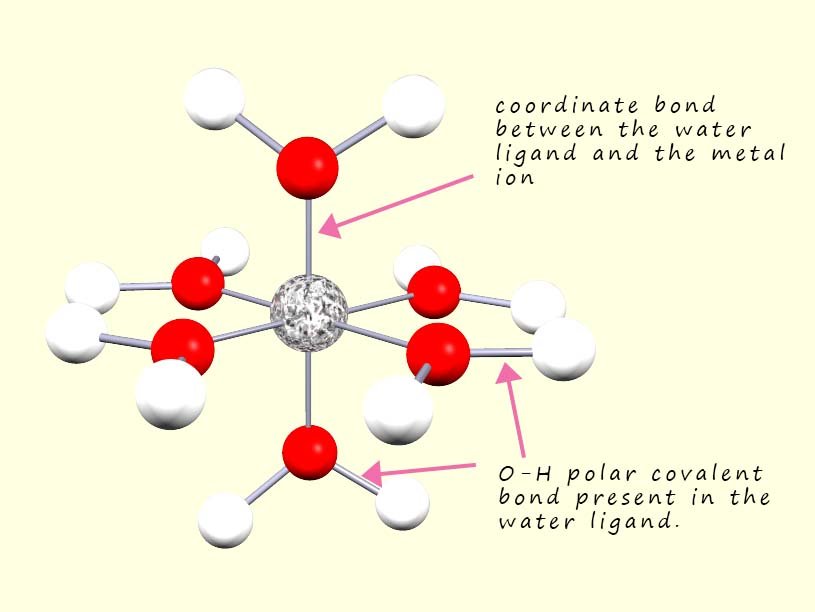
 Metal complexes involve two main types of bonding: coordinate bonds between the central metal ion and the ligands and covalent bonds within the ligands themselves. The image opposite shows a typical hexaaqua metal complex, here six water molecules have used one of their lone pairs of electrons to act as ligands or Lewis bases to form six coordinate bonds to completely surround the central metal ion in an octahedral arrangement. The other bonds present within this complex are simply the covalent bonds present between the oxygen and hydrogen atoms in the water ligands.
Metal complexes involve two main types of bonding: coordinate bonds between the central metal ion and the ligands and covalent bonds within the ligands themselves. The image opposite shows a typical hexaaqua metal complex, here six water molecules have used one of their lone pairs of electrons to act as ligands or Lewis bases to form six coordinate bonds to completely surround the central metal ion in an octahedral arrangement. The other bonds present within this complex are simply the covalent bonds present between the oxygen and hydrogen atoms in the water ligands.
So when a metal complex undergoes a chemical reaction then either the coordinate bonds are broken or the covalent bonds present within the attached ligands are broken.
If the O-H bonds present within the attached water ligands are broken then this is called an acidity or hydrolysis reaction. However here we will focus on ligand substitution or exchange reactions which take place when the coordinate bonds between the metal ion and the attached ligands are broken. As the name suggests these reactions occur when for example the water ligands present in a hexaaqua complex are replaced by one or more different ligands, for example the image below shows two common ligand substitution reactions.
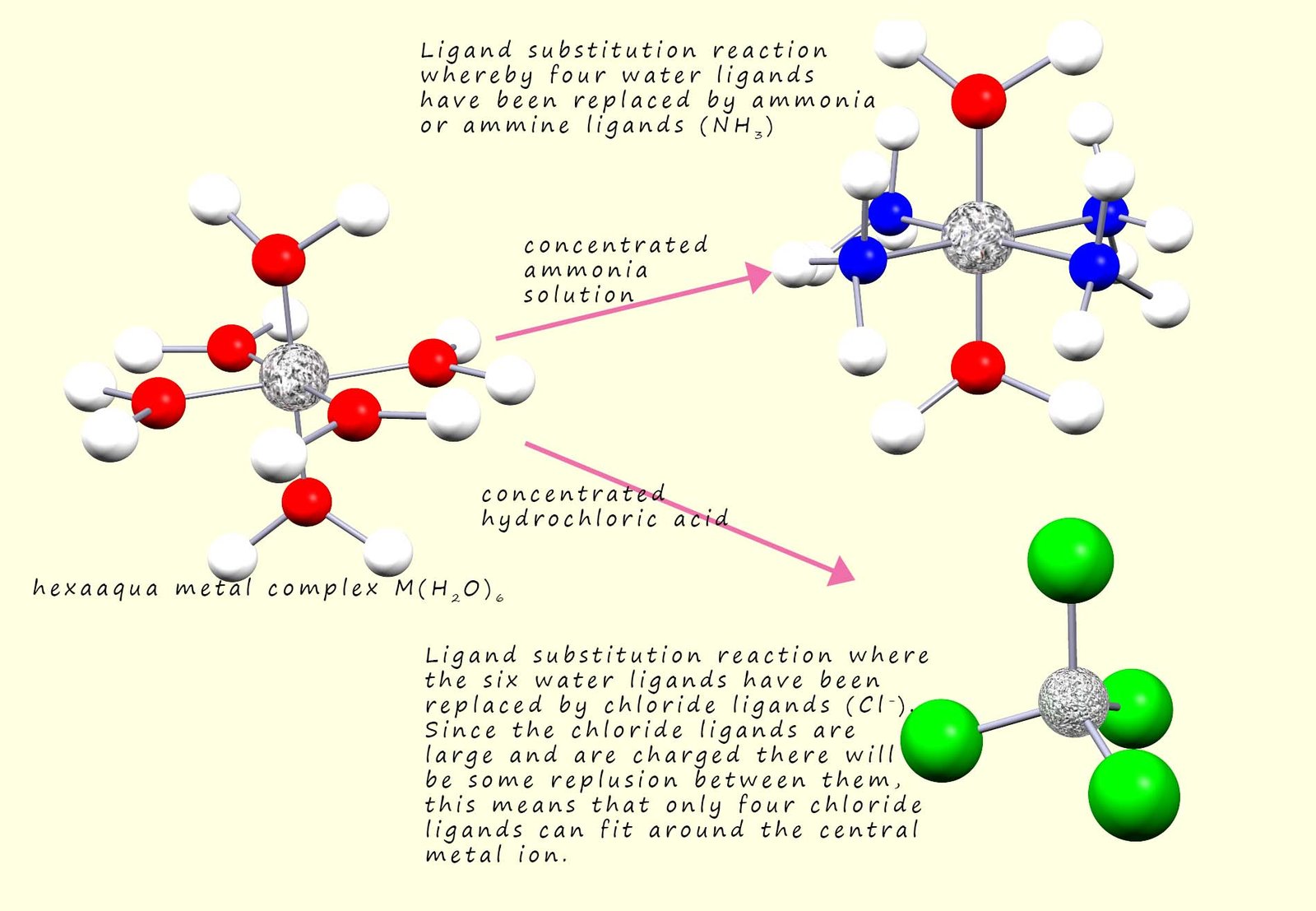
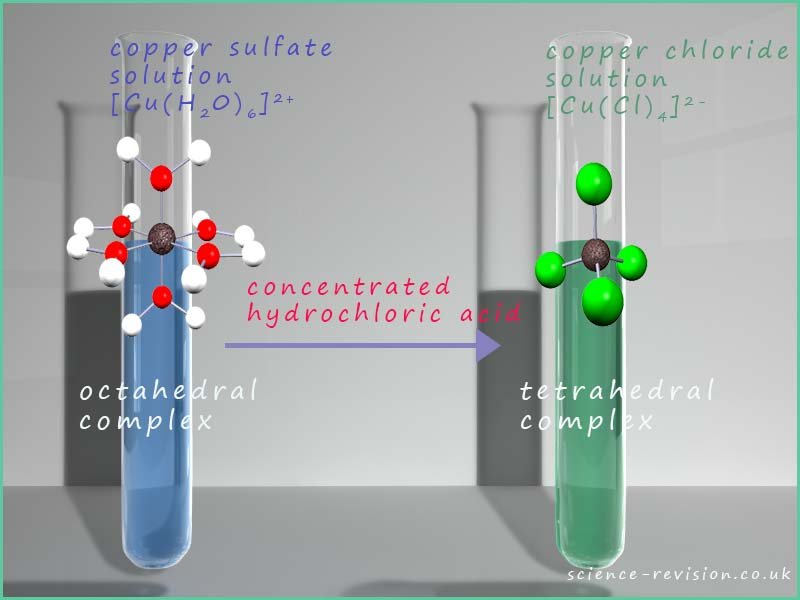
It is very easy to change the ligands present in a transition metal complex, these ligand exchange or ligand substitution reactions often involve a colour change or a change in the coordination number of the metal ion present in the complex, as an example consider the reaction that occurs when concentrated hydrochloric acid is added to a blue copper(II) sulfate solution. Now one of the main species present in the copper(II) sulfate solution will be the hexaaquacopper(II) ion [Cu(H2O)6]2+. Additional of concentrated hydrochloric acid will result in the substitution of the six water ligands surrounding the copper ion with four chloride ligands (Cl-) resulting in the formation of the tetrachlorocuprate(II) complex ion [Cu(Cl4)2-. This ligand substitution reaction results in the copper(II) sulfate solution changing colour from blue to green and also a change in coordination number of the copper ion as the geometry of the complex changes from octahedral to tetrahedral. This is outlined in the image below:
We can represent this reaction using the equation below:
Now a dilute solution of copper (II) chloride is light blue in colour while a concentrated solution is green, we can use Le Chatelier's principle to explain this observation. In a dilute solution the hexaaquacopper(II) ion- [Cu(H2O)6]2+is the main ion present and this ion is blue. However as the concentration of the chloride ion (Cl-) is increased then according to Le Chatelier's principle the position of equilibrium in the above equation will shift to the right and the concentration of the tetrachlorocuprate(II) ions -[Cu(Cl)4]2- will increase, now these ions are yellow in colour and the solution will now appear green because it is an equilibrium mixture of the blue hexaaquacopper(II) ions and the yellow tetrachlorocuprate(II) ions and this mixture of yellow and blue ions appears green!
The image below shows a series of ligand substitution reactions, as you move from the hexaaquacopper(II) solution on the left towards the copper EDTA complex on the right the complexes become more stable. The relative stability of each of the complexes in the image can be worked out by simply referring to the equilibrium constants for each of the ligand substitution reactions that take place relative to the hexaaquacopper(II) ion.
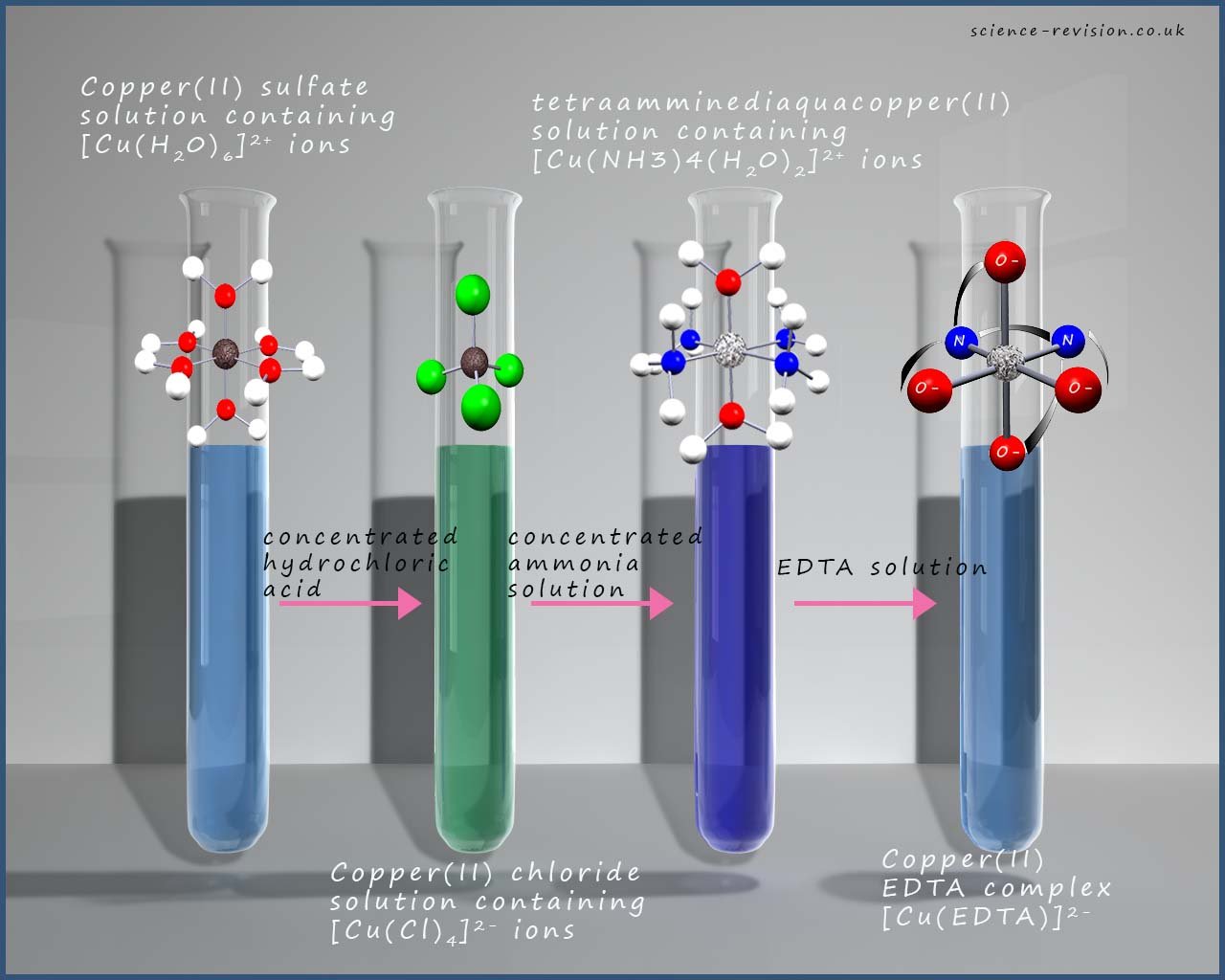
So for example the two equations below represent the formation of the tetrachlorocuprate(II) ions-[Cu(Cl)4]2- and the tetraamminediaquacopper(II) ions [Cu(NH3)4(H2O)2]2+(aq) , now the equilibrium constant for the formation of the tetraamminediaquacopper(II) ion is much larger than for the tetrachlorocuprate(II) ions and since both reactions start from the hexaaquacopper(II) ion the larger equilibrium constant suggests the position of equilibrium lies more to the right hand side and so favours the product. This suggests that the reaction with the higher equilibrium constant contains the more stable complex ion. However there is another factor that can help us explain why the complexes in the image because more stable as we move from the left-hand side to the right-hand side of the image.
There is another factor that we should consider when dealing with ligand substitution reactions, that is the entropy factor. The two equations above involved the exchange or substitution of a monodentate ligand. However when a monodentate ligand is replaced by a bidentate ligand such as ethylenediamine (en) or a polydentate ligand such as EDTA then the equilibrium constant for these reactions is very large, much larger than those where one monodentate ligand is exchanged or substituted for another monodentate ligand. As an example consider the equation below which involves the ligand exchange or substitution of six water ligands for three ethylenediamine (en) ligands.
In this reaction there are 4 "particles" on the reactants side of the equation and 7 "particles" on the products of the equation, that is there is an increase in entropy as this reaction takes places. In these reaction which involve the formation of chelates, that is complexes where monodentate ligands are replaced by bidentate and polydentate ligands, the enthalpy changes are usually not that large, so recall that for a reaction to be feasible the value of the Gibbs free energy (ΔG) must be zero or negative.
ΔG = ΔH - TΔS
Since the entropy changes for the formation of chelates is always large and positive this will contribute to a very large negative value for the TΔS term, this value always outweighs the value of the enthalpy changes for these reactions and leads to a very large negative value for ΔG. This large increase in entropy is a major factor in the large equilibrium constants for these reactions and the stability of the resulting complexes. This increase in stability of these complexes as evidenced by their large equilibrium constants and the large positive values for the entropy changes of the reaction is often called the chelate effect.
As another example of a ligand substitution reaction consider the reaction of hexaaquacobalt(II) ions- [Co(H2O)6]2+ (aq) with concentrated hydrochloric acid. This reaction is similar to the one above using copper(II) ions. A solution of hexaaquacobalt(II) ions- [Co(H2O6)]2+ is pink and when concentrated hydrochloric acid (HCl) is added to this solution it turns blue as the tetrachlorocobaltate(II) ions are formed-[Co(Cl4)]2-. An equation for this ligand substitution reaction is shown below:
Like the reaction of hexaaquacopper(II) ions with hydrochloric acid this reaction is reversible and also involves a change in the geometry of the complex from octahedral to tetrahedral, this is outlined in the image below. We can use Le Chatelier's Principle to explain what happens when an excess of chloride ions (Cl-) from the hydrochloric acid is added to the hexaaquacobalt(II) ions, the addition of extra chloride ions (Cl-) will push the position of equilibrium towards the product side of the equation and cause a change in colour from pink to blue as the [Co(Cl4)]2- complex ion forms, however again applying Le Chatelier's Principle if this solution is then diluted by the addition of water this will push the position of equilibrium back towards the reactants and results in a colour change from blue back to pink.
![Image shows the colour change and geometry change as hexaaquacobalt(II) [Co(H2O)6]2+ reacts with concentrated hydrochloric acid to form tetrachlorocobaltate(II) ion [Co(Cl)4]2-.](images/cobalt chloride ligand exchange.jpg)
Thiocyanate ions have the formula SCN- and this ion has a resonance stabilised structure as shown in the image below:
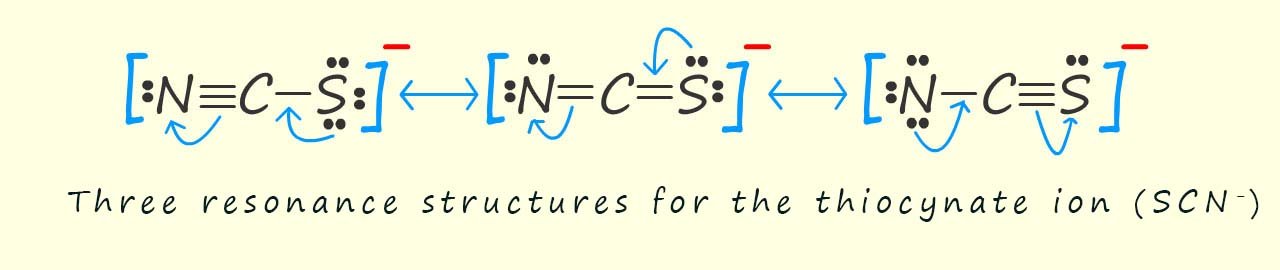
Now you may notice that in all of the resonance hybrid structures for the thiocyanate ion that the nitrogen and sulfur atoms always have at least lone pair of electrons, now the thiocyanate ion (SCN-) is often referred to as an ambidentate ligand meaning that it can form a coordinate bond to a metal ion through two different donor atoms, in this case one of the lone pairs of electrons on the sulfur atom is capable of forming a coordinate bond to a metal ion and the nitrogen atom present in the thiocyanate ligand can also use a lone pair of electrons to form a coordinate bond to a metal ion. When this ligand forms a coordinate bond through the sulfur atom , the ligand is referred to as thiocyanato and when it forms a coordinate bond to a metal ion through the nitrogen atom it is referred to as a isothiocyanato ligand.
The thiocyanate ligand (SCN-) can be used as a test for the presence of the iron(III) ion. A solution of iron(III) ions has a pale yellow colour and even if the smallest amount of thiocyanate ions are added to a solution containing Fe3+ ions a deep blood red solution containing the complex ion pentaaquathiocyanatoiron(III)- [Fe(H2O)5(SCN)]2+ is formed. The thiocyanate ions undergo a ligand substitution reaction with the hexaaquairon(III) ions -[Fe(H2O6)]3+ as shown in the equation and image below:
![Image to show how the blood red complex ion pentaaquathiocyanatoiron(III)- [Fe(H2O)5(SCN)]2+ is formed on by the addition of thiocyante ions to a solution containing iron(III) ion.](images/thiocyanate.jpg)
It is worth mentioning that Fe2+ ions do not form a coloured complex with thiocyanate ions, so as well as being a test for the presence of Fe3+ ions thiocyanate ions can also be used to distinguish between Fe2+ and Fe3+ ions.