This page deals with the addition of bases such as sodium hydroxide to solutions containing metal ions. To fully understand the ideas covered here it is important that you are very familiar with the acidity or hydrolysis reactions that take place in aqueous solutions containing metal ions with a 2+ and 3+ charge. If you need to review these ideas then click here or in the link at the top of the page. I have included a brief summary of the acidity or hydrolysis reaction below for you to review.
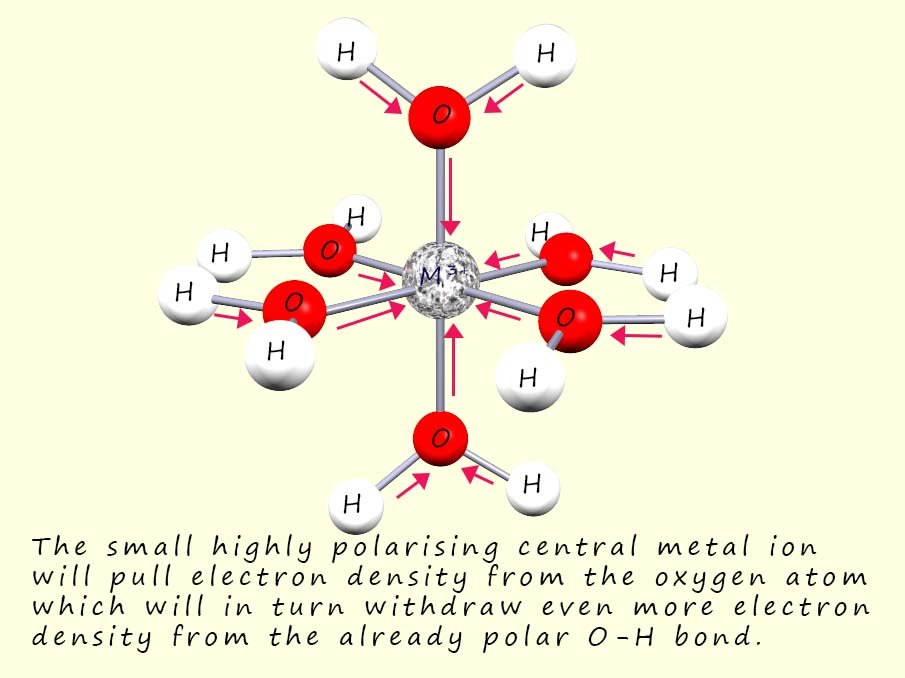 When a transition metal ion forms a complex ion the small highly charged central metal ion will polarise or distort the electron density in the water molecules bonded to it, that is it will pull or withdraw electron density from the water ligands. The M-O bond will become polarised with the metal ion withdrawing electron density from the oxygen atom present in the water molecule. The larger the size of the charge on the central metal ion and the smaller its radius then the more it will be able to polarise and withdraw electron density towards it, this will weaken the O-H bond in the water ligand and make it easier for the loss of a hydrogen ion (H+) to occur, this is outlined in the image opposite; where the red arrows indicate the direction of electron flow towards the central metal ion, in this case a metal ion with an oxidation state of 3+.
When a transition metal ion forms a complex ion the small highly charged central metal ion will polarise or distort the electron density in the water molecules bonded to it, that is it will pull or withdraw electron density from the water ligands. The M-O bond will become polarised with the metal ion withdrawing electron density from the oxygen atom present in the water molecule. The larger the size of the charge on the central metal ion and the smaller its radius then the more it will be able to polarise and withdraw electron density towards it, this will weaken the O-H bond in the water ligand and make it easier for the loss of a hydrogen ion (H+) to occur, this is outlined in the image opposite; where the red arrows indicate the direction of electron flow towards the central metal ion, in this case a metal ion with an oxidation state of 3+.
The withdrawal of electron density from the already polar O-H bond in the water ligand allows for the loss of a hydrogen ion (H+), which obviously results in the formation of an acidic solution. Now it is important to realise that the hydrogen ion (H+) does not simply just "fall off" the water ligand, instead another water molecule will act as a Brønsted-Lowry base and remove one of the hydrogen ions (H+) to form a hydronium or hydroxonium ion (H3O+) We can represent this as using the equation below, here the complex ion contains a metal ion with a 3+ oxidation state:
The H3O+ ion is called the hydronium or hydroxonium ion and it is usually simply represented as H+ in many chemical equations, you can simplify the equation above to give:
However you should bear in mind that the hydrogen ion (H+) in the above equation is actually a hydronium ion (H30+) that forms when a water molecule acts as a base and removes a hydrogen ion (H+) from the [M(H2O)6]3+ complex as outlined in the image below:
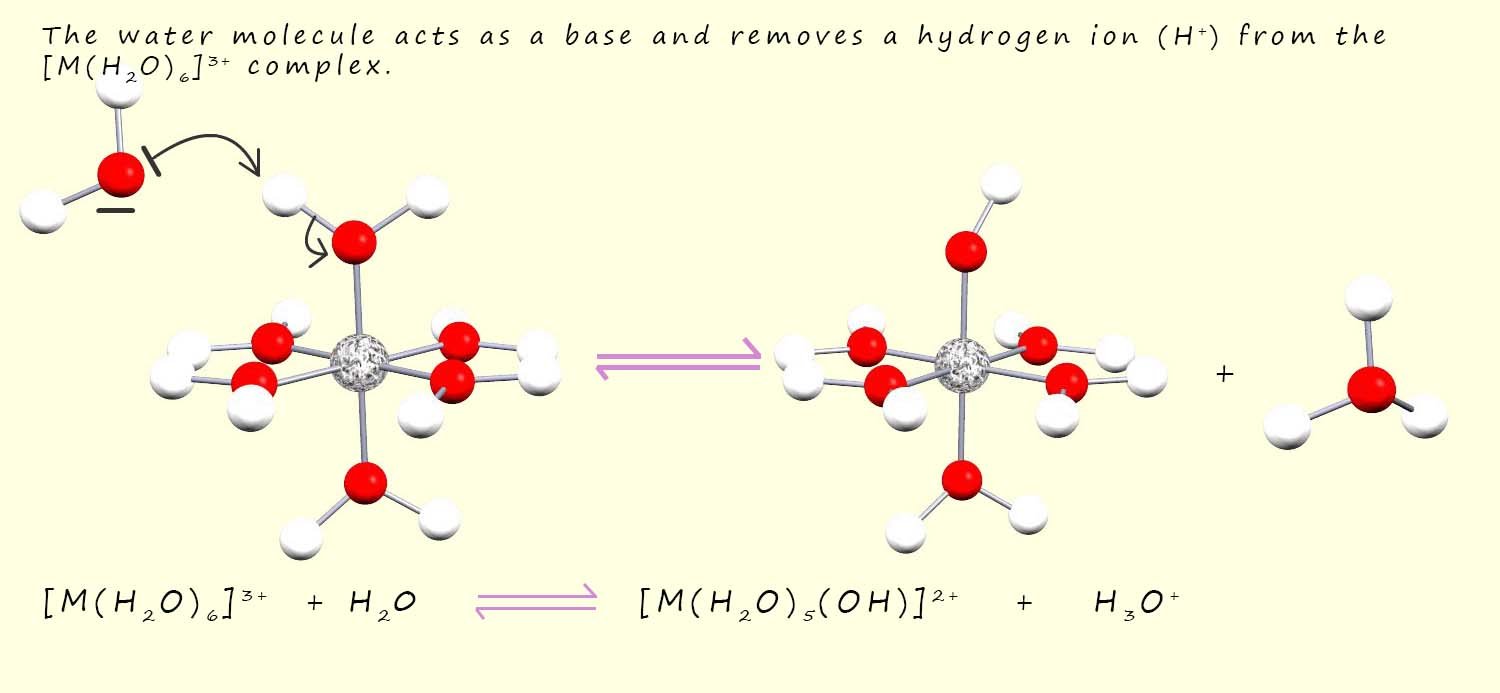
In this equilibrium reaction a water molecule bonded to the metal ion has been split or broken up into a hydrogen ion (H+) ion and a hydroxide ion (OH-) ion so for this reason the reaction is often referred to as a hydrolysis reaction but it is also commonly referred to as an acidity reaction because a hydronium ion (H3O+) is formed.
Now there are obviously six water ligands surrounding the central metal ion in a hexaaqua complex and so far we have only considered the loss of one hydrogen ion (H+) from one of the water ligands, however you can get the loss of additional hydrogen ions (H+) from the remaining water ligands:
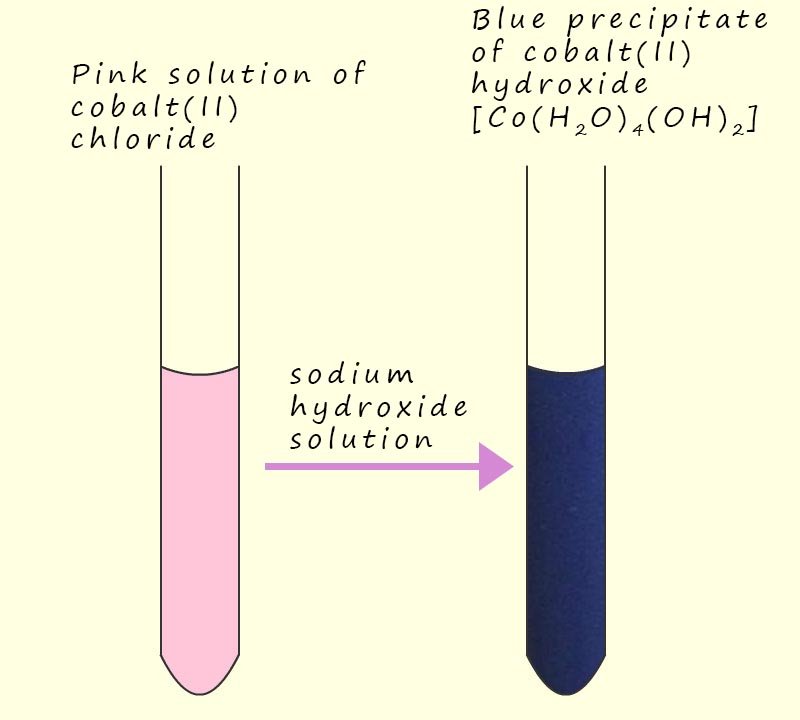
In the acidity or hydrolysis reactions of aqueous solutions containing metal (II) and metal (III) ions the water molecules can act as a Brønsted-Lowry base and remove a hydrogen ion (H+) from a water ligand in the metal complex. In the case of an aqueous solution containing a metal(II) ion the acidity reactions taking place are shown below. In the first equation below a water molecule will remove a hydrogen ion (H+) from the hexaaqua complex [M(H2O)6]2+(aq) to form [M(H2O)5(OH)2]+(aq), as shown in the equation below:
While the equation below shows the removal of a second hydrogen ion (H+) from a different water ligand to form a neutral solid precipitate of [M(H2O)2(OH)2](s):
However you should bear in mind that the main species present in a solution of metal (II) ions will be the hexaaqua complex [M(H2O)5(OH)]2+(aq) and no precipitate will likely be observed unless the concentration of the solution is increased. In this case we can simply use Le Chatelier's principle to say that as the concentration of the hexaaqua ion ([M(H2O)5(OH)]2+(aq)) increases the position of equilibrium will shift to the right.
However water is not a particularly strong base so what would happen if we added a stronger base such as sodium hydroxide to a solution containing a metal (II) ion such as a blue copper (II) sulfate solution? Well there are two possible reactions that can take place:
If we consider only the first hydrolysis reaction that takes place, since this will contain the main ions present then we can show this as:
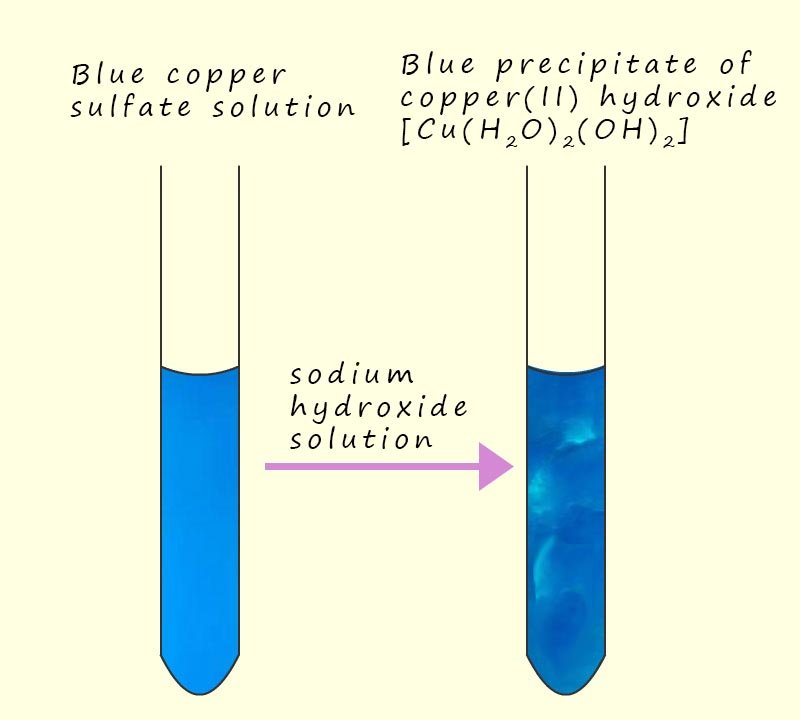 Well the basic hydroxide ion (OH-) will simply react with one of the acidic hydrogen atoms present on a water ligand to form:
Well the basic hydroxide ion (OH-) will simply react with one of the acidic hydrogen atoms present on a water ligand to form:
And if more sodium hydroxide is added then one more acidic hydrogen ion (H+) will be removed from a water ligand in the [Cu(H2O)5(OH)]+ complex to form the insoluble precipitate of copper hydroxide; as shown in the image opposite:
Now the addition of a base such as sodium hydroxide to form a solid precipitate is a reversible reaction and if a strong acid such as nitric acid is added then the solid precipitate of copper(II) hydroxide formed above will simply dissolve as the hexaaquacopper(II) ion is reformed, this again is simply a case of applying Le Chatelier's principle to a reversible reaction, the equation below shows how the solid precipitate of copper(II) hydroxide forms and if a nitric acid solution is added then it will simply increase the concentration of the hydronium ions (H3O+) ions present, which will then force the position of equilibrium to shift towards the reactants. If enough nitric acid is added then this will ultimately lead to the formation of the hexaaquacopper(II) complex [Cu(H2O)6]2+(aq).
The same sequence of reactions shown above for metal(II) ions will also occur with compounds containing metal ions with a charge of 3+ in aqueous solutions when a strong base such as sodium hydroxide added. The sequence of equations below show the step by step process as one hydrogen ion (H+) after another is removed from a hexaaqua complex containing a metal (III) ion. Each step involves the addition of more hydroxide ions (OH-) until the neutral complex [M(H2O)3(OH)3](s) forms, at this point since the complex has no charge a solid insoluble precipitate will form.
However depending on the particular metal ion if more sodium hydroxide is added then the solid precipitate formed will dissolve as one or more hydrogen ions are removed from it in a stepwise manner. The equations below show in a stepwise manner the complex ion formed as more sodium hydroxide is added until finally the hexahydroxo complex is formed.
All solutions of transition metal ions will form coloured precipitates when a base such as sodium hydroxide is added, for example the image below show the coloured precipitates formed when sodium hydroxide solution is added to solutions of Cu2+, Fe2+, Fe3+ and Al3+ ions:
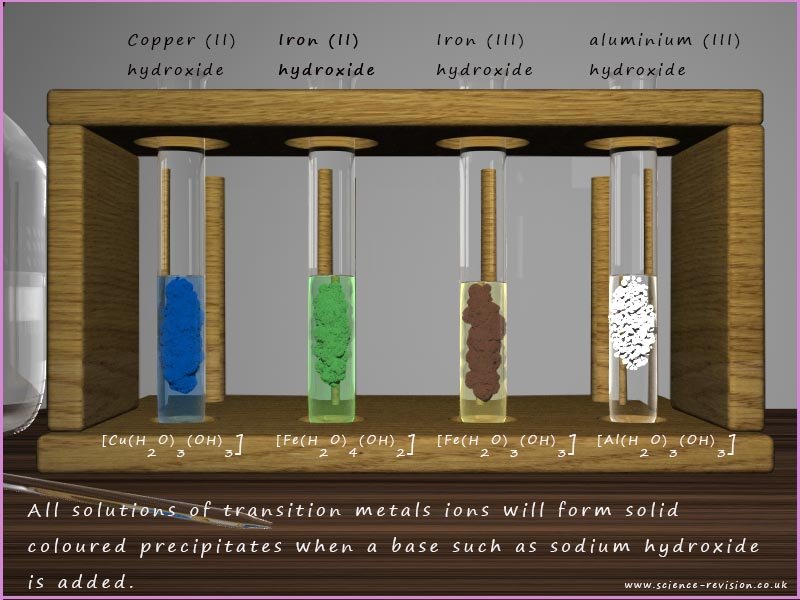
Most but not all metal hydroxides formed by the addition of an excess of the strong base sodium hydroxide
will react to form anionic complexes, that is complexes with a negative charge. As an example consider the addition of sodium hydroxide solution to an aqueous solution containing aluminium(III) ions (Al3+), as you might expect initially a white or colourless precipitate of aluminium hydroxide [Al(H2O)3(OH)3] forms; remember that aluminium is not a transition metal so the precipitate formed will be white or colourless. However as more sodium hydroxide solution is added the precipitate then dissolves to form a colourless solution of tetrahydroxoaluminate(III) ions; [Al(OH)4]-; an equation to show these reactions is shown below:
Additional of more sodium hydroxide will result in the white solid precipitate dissolving as the hydroxide ion (OH-) removes one more hydrogen ions (H+) from a water ligand and the loss of two other water ligands will give the anionic complex:
As we have seen above addition of sodium hydroxide solution to an aqueous solution containing a aluminium(III) ions (Al3+) results in the formation of an insoluble precipitate of the metal hydroxide [Al(H2O)3(OH)3] followed by the formation of the soluble anionic complex [Al(OH)4](s)- if an excess of sodium hydroxide is added. However we also saw above that it is possible to reverse these reactions by simply adding an acid such as nitric acid. Addition of nitric acid to a solution of tetrahydroxoaluminate(III) ions- [Al(OH)4]- will initially result in the formation of a white precipitate of [Al(H2O)3(OH)3] which will quickly dissolve to form the hexaaluminium(III) complex- [Al(H2O)6]3+ complex. Metal hydroxides such as aluminium oxide that can react with both alkalis and acids in this manner are called amphoteric oxides. This system of reversible reactions can be shown as:
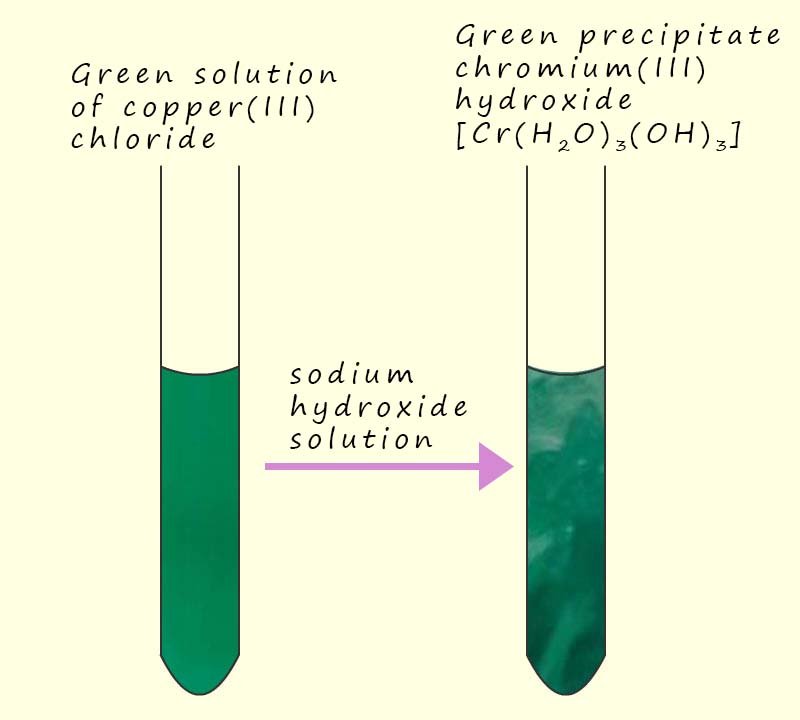 Solutions containing the chromium(III) ion (Cr3+) will react in a similar way to that described for Al3+ ions above. If sodium hydroxide solution is added to a green solution of chromium(III) chloride the a green precipitate of chromium hydroxide [Cr(H2O)3(OH)3)] will form, this is shown in the image opposite.
Solutions containing the chromium(III) ion (Cr3+) will react in a similar way to that described for Al3+ ions above. If sodium hydroxide solution is added to a green solution of chromium(III) chloride the a green precipitate of chromium hydroxide [Cr(H2O)3(OH)3)] will form, this is shown in the image opposite.
If an excess of sodium hydroxide is added then a similar series of reactions that occurred with the aluminium hydroxide precipitation reaction will occur. The green precipitate of chromium(III) hydroxide will dissolve in an excess of sodium hydroxide to form the hexahydroxochromate(III) ion -[Cr(OH)6]3+, an equation for this reaction is shown below:
And if the hexahydroxochromate(III) solution is now acidified using hydrochloric acid then as before the reaction above will be reversed, so initially a green precipiate of chromium (III) hydroxide [Cr(H2O)3(OH)3)] will be formed but this will then dissolve as more acid is added to form the original green solution of hexaaquachromium(III)chloride.