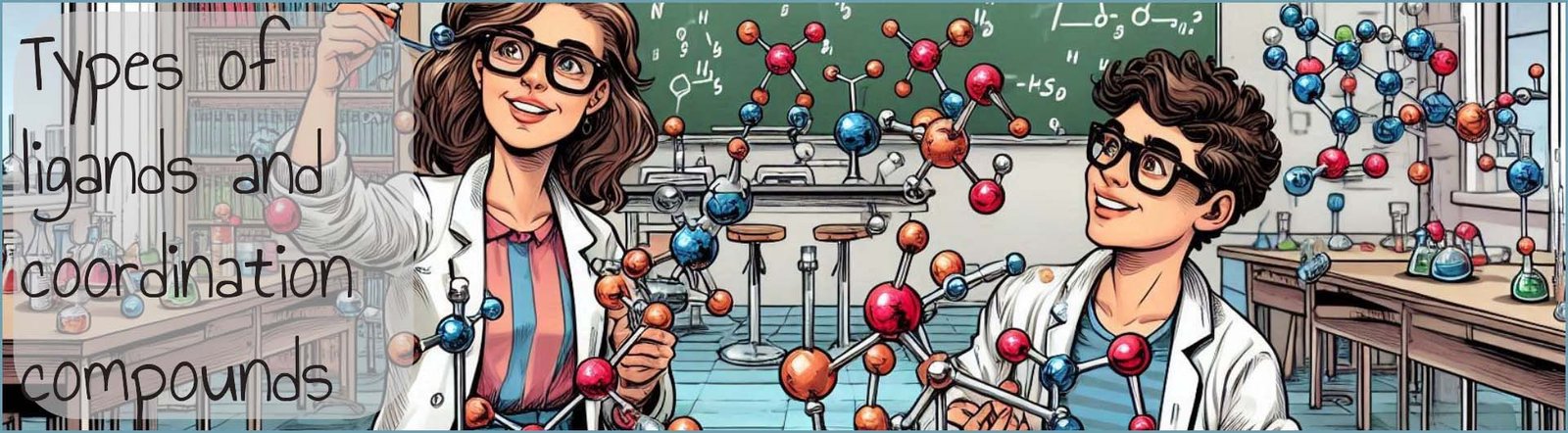
Ligands as you may recall are Lewis bases, that is they contain at least one lone pair of electrons that they are able to use to form a dative or coordinate bond to a metal atom or ion, which acts as a Lewis acid. The majority of ligands that you are likely to meet will be unidentate or monodentate ligands, that is a ligand that uses only one pair of electrons to form a coordinate bond to a metal ion or atom, now some monodentate ligands such as water molecules may contain more than one lone pair of electrons but they are only able to use one of these lone pairs of electrons to form a coordinate bond to a metal atom or ion; examples of common monodentate ligands are shown below:
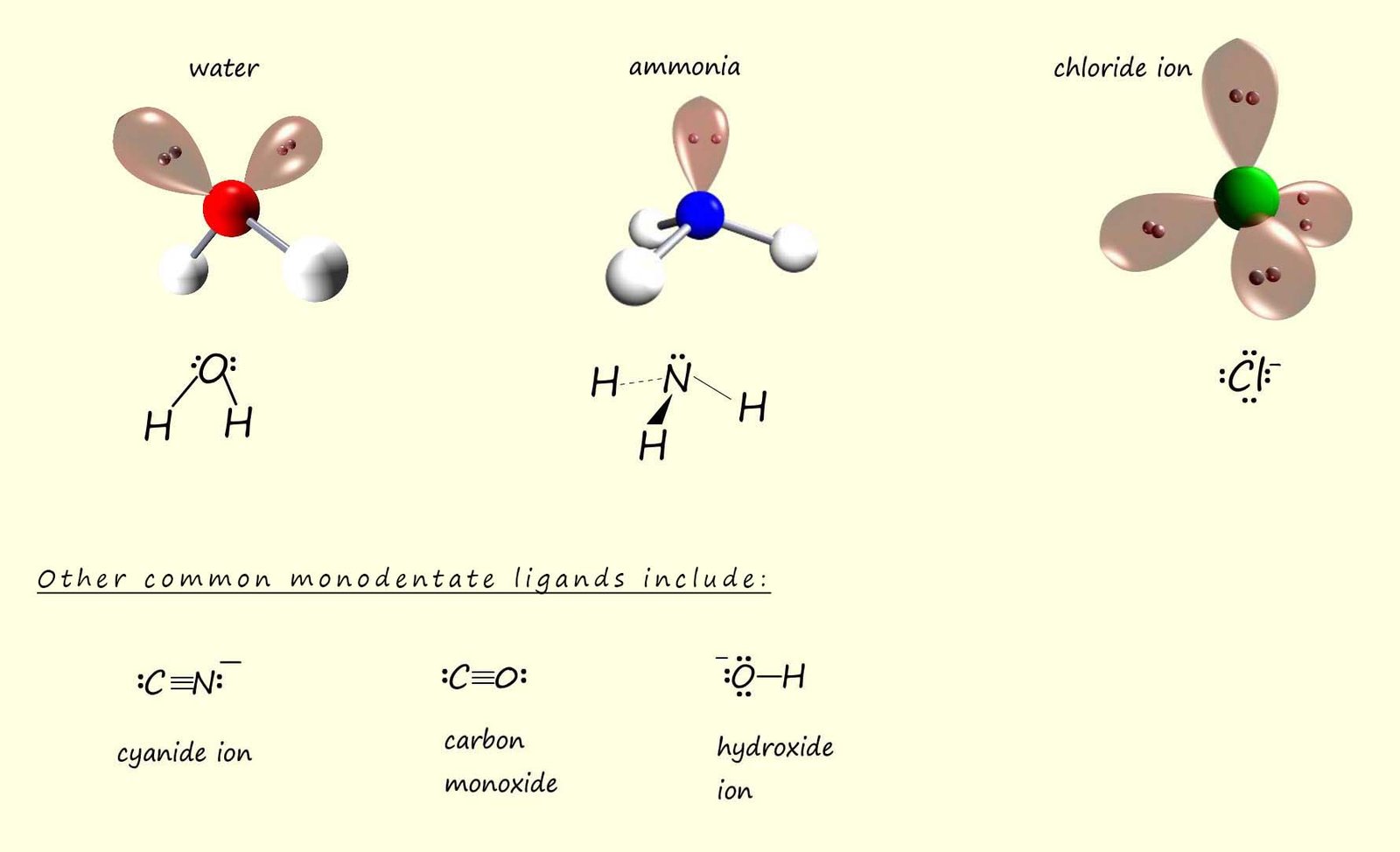
While all the ligands shown above are monodentate ligands, that is ligands that only bond to a metal atom or ion using one lone pair of electrons there are other types of ligands that use more than one lone pair of electrons to form multiple coordinate bonds to a central metal atom/ion in a complex, these ligands are called polydentate ligands. One example of such a ligand is ethylenediamine or ethane-1,2-diamine, the structure of this bidentate ligand is shown below. A bidentate ligand is one that forms two coordinate bonds to a metal atom or ion.
Ethylenediamine is a small molecule which contains two nitrogen atoms, these nitrogen atoms can use their lone pairs of electrons to form two coordinate bonds to a metal atom or ion, hence they are called bidentate ligands. An example of a complex containing this bidentate ligand is the octahedral [Co(en)3]3+ complex; which is shown in the image below; you may notice that the formula for the ethylenediamine ligand (H2NCH2CH2NH2) is often simply shortened to "en". To try and help you visualise how the nitrogen atoms in the ethylenediamine ligands bond to the central cobalt ion I have shaded each of the nitrogen atoms in the three separate ethylenediamine ligands a slightly different colour.
![3d model and shorthand model of the [Co(en)3]3+ complex](images/ethylenediamine complex.jpg)
However it can be difficult to visualise the structure of this complex from the 3d model so a simplified version of the octahedral shaped complex is also shown. It is clear from this simplified structure that each of the ethylenediamine ligands is forming two coordinate bonds to the central cobalt ion.
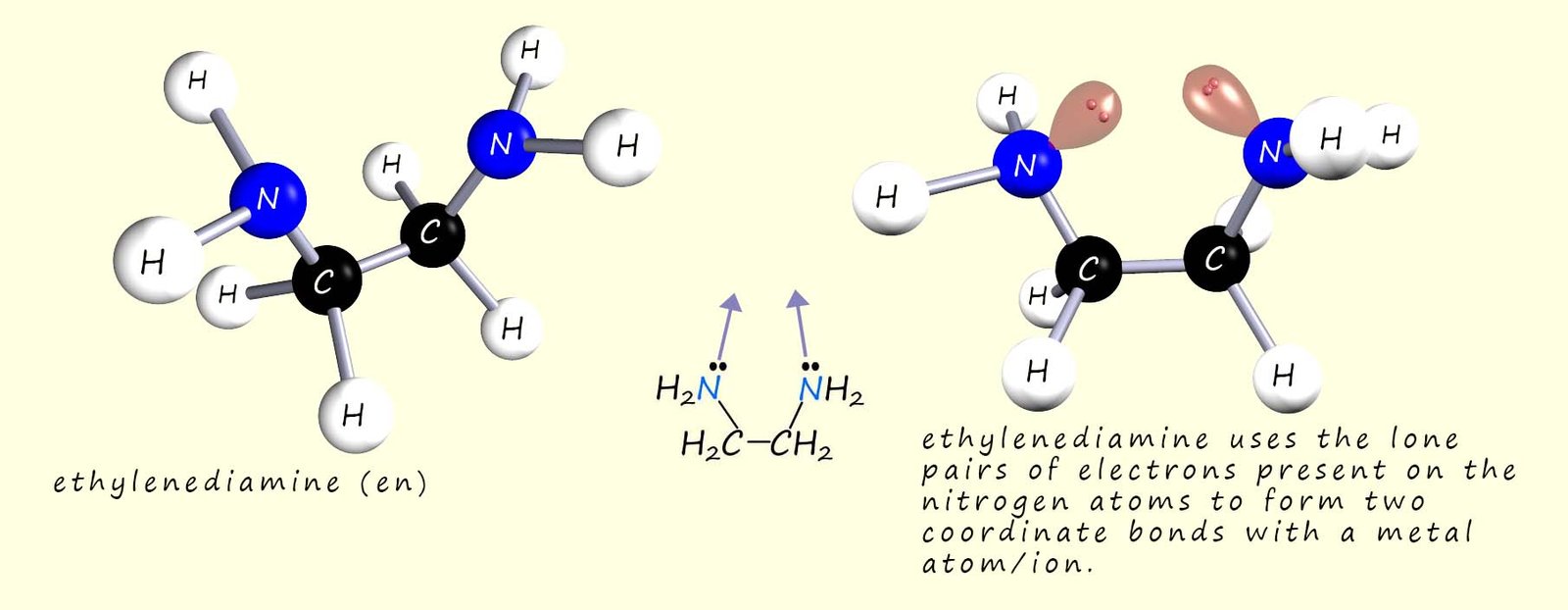
Another bidentate ligand that you may meet is the ethanedioate ion (C2O42-) or you may see it called by its traditional name which is the oxalate ion, a 3d model and displayed formula for this bidentate ligand is shown below. Like ethylenediamine it can form two coordinate bonds but this time it uses one of the lone pairs of electrons on an oxygen atom instead of a nitrogen atom.
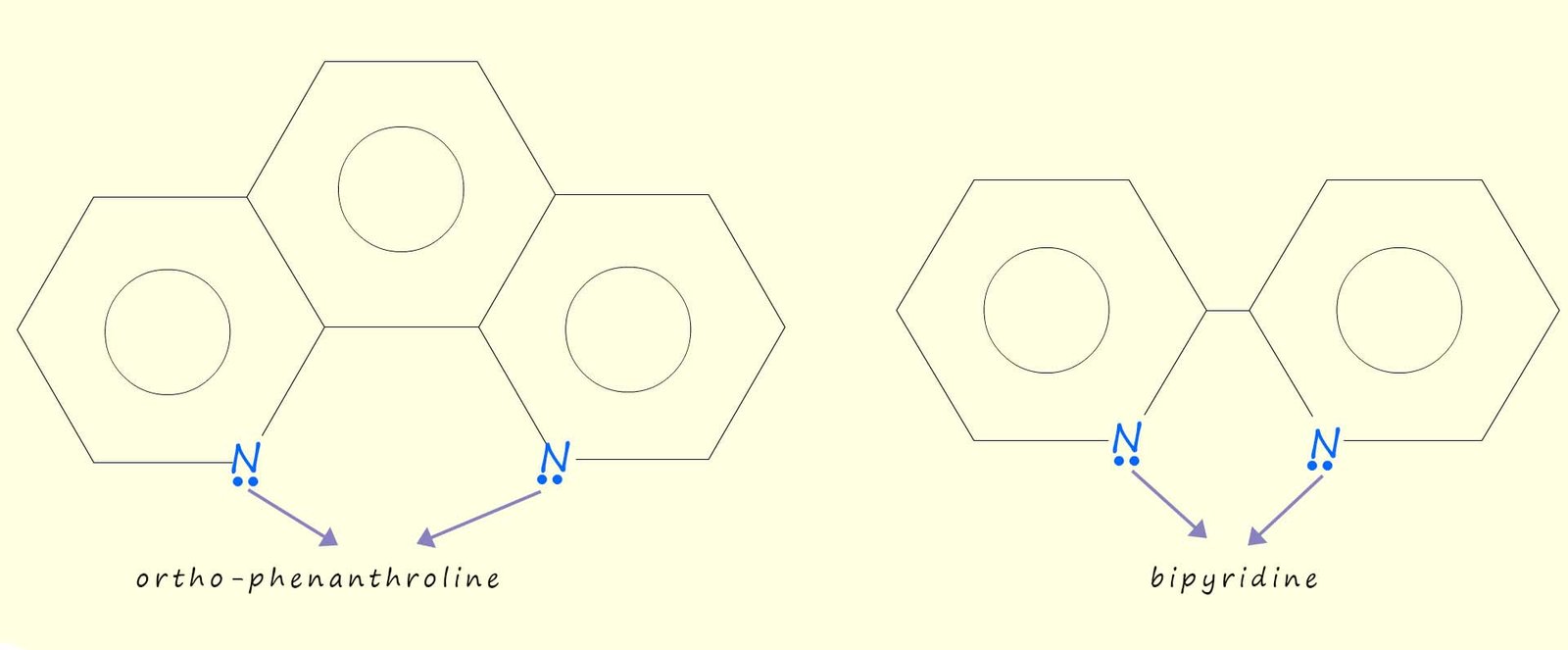
Two aromatic bidentate ligands that you may also meet are ortho-phenanthroline and bipyridine; whose name is often shortened to bipy are shown in the image below, they are similar to the ethylenediamine bidentate ligand in that they also use two nitrogen atoms to form coordinate bonds to a metal ion in a complex.
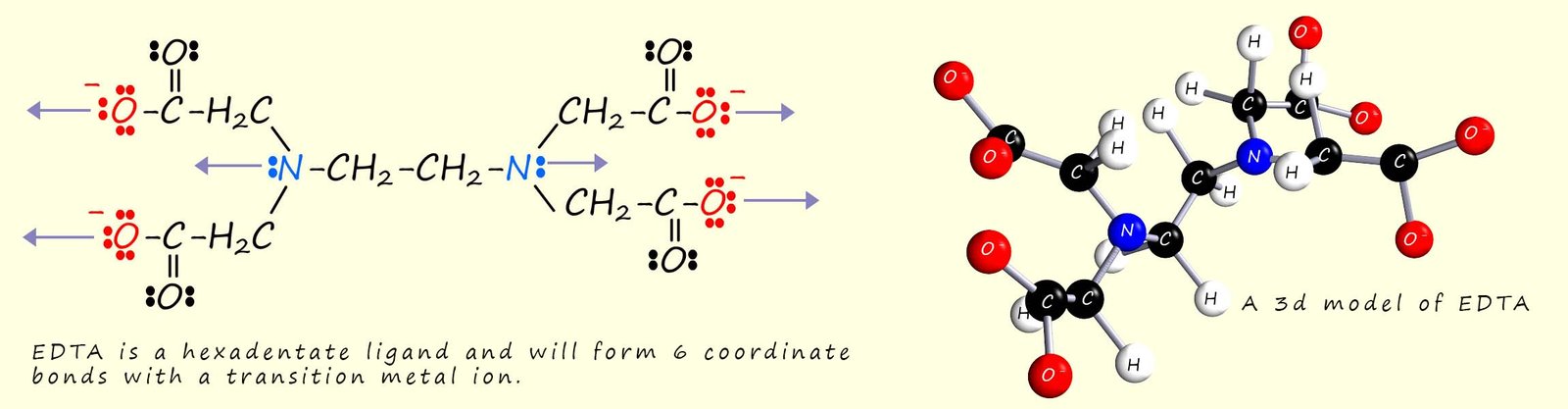
EDTA4- (ethylenediaminetetraacetic acid) is a hexadentate ligand; that is it will form six coordinate bonds to a metal ion. The structure of EDTA is shown below and the purple arrows shown in the image indicate the six lone pairs of electrons in the EDTA ligand that will bond to the metal ion.
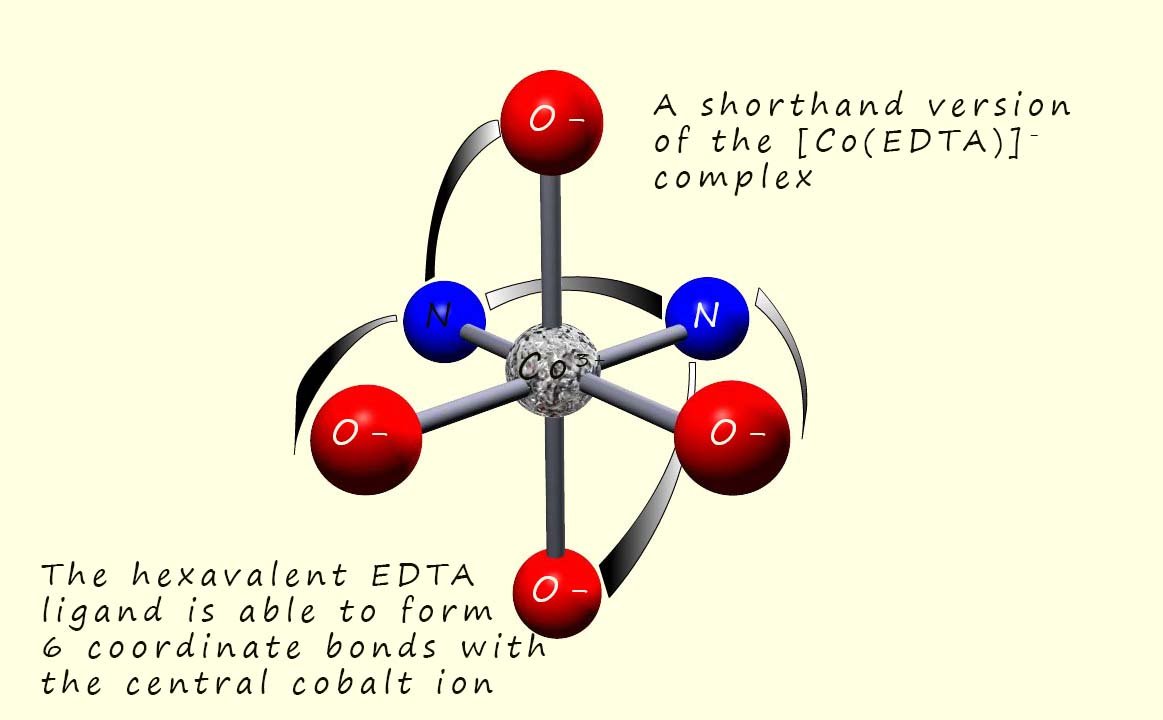
The EDTA molecule is able to wrap itself around the central metal ion in a complex and form six coordinate bonds with it, the image below shows the shortened formula of the EDTA ligand forming 6 coordinate bonds with a central cobalt ion.
Ligands such as chloride (Cl-) ions, cyanide ions (CN-), fluoride ions (F-) and neutral molecules such as water (H2O) or ammonia (NH3)
are monodentate or unidentate ligands and used only one lone pair of electrons to form a coordinate bond to a central metal atom/ion in a transition metal complex. However ligands such as ethylenediamine (en); a bidentate ligand and EDTA; a hexadentate ligand are able to form multiple bonds to a central metal ion, these ligands which are able to form more than one coordinate bond to the metal ion are often referred to as chelating agents or chelates (from the Greek word "chele" meaning claw).
These chelating ligands are able to form complexes which are much more stable than those formed with simple monodentate ligands, this is seen in the equilibrium contants for the formation of these complexes, the equilibrium constants for the formation of complexes involving chelating ligands are very large in comparison to those for monodentate ligands. This added stability found in these complexes is often referred to as the chelating effect.

Many metals such as lead, mercury and cadmium are toxic and can have serious detrimental effects on the human nervous system and can cause various neurological damage as well as kidney damage and serious respiratory problems, however it is possible to remove these metals from the tissues in the body where they have built up by treatment with a chelating agent such as EDTA. Here the EDTA is usually injected into the bloodstream where it will bind to these metal ions in the body tissues and form very stable complexes which are water soluble, since these complexes are now water-soluble they can be easily filtered by the kidneys and excreted in urine.
Salad dressings, mayonnaises, sauces as well as processed meats and many canned foods have EDTA added as a preservative. The presence of metals such as magnesium, calcium, copper and zinc which are naturally found in soils and water end up in many of these foods and the presence of these metals will help speed up the oxidation processes in the foods which will cause the foods to spoil and lose their distinctive tastes, flavours and colour. EDTA can form stable complexes with many of these metal ions and so prevent these oxidation reactions in the foods from taking place and so extend their shelf life.
EDTA and other chelating agents are also added to many boilers and heating equipment, here the EDTA will remove any metals ions present and so prevent the build up of scale and also prevent corrosion both of which will reduce the efficiency and life of the equipment.
EDTA and other chelating agents are used in paper making industry where it removes metal ions from the paper pulp which results in "whiter" and brighter paper.
Many important biological molecules such as the protein haemoglobin; which is responsible for the transport of oxygen around the body and the pigment chlorophyll found in the leaves of plants are based on the porphine conjugated ring structure shown in the image below; now porphine is a tetradentate ligand; that is one that is able to form four coordinate bonds. When porphine forms coordinate bonds to a metal ion the hydrogen atoms attached to the nitrogen atoms in the ring structure are lost and this will then allow the four nitrogen atoms to form four coordinate bonds.
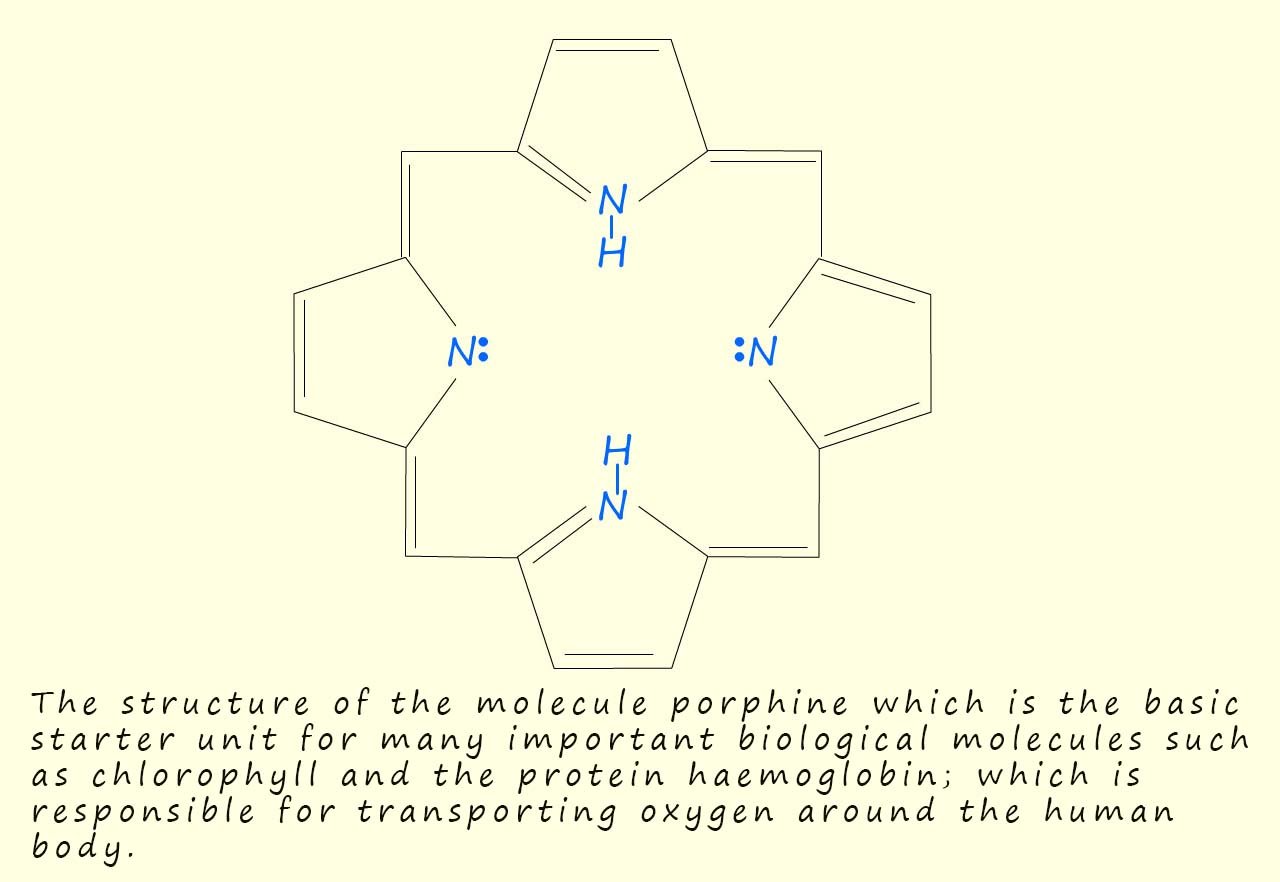
You may notice that porphine has a conjugated ring system, that is one which contains five membered rings linked by methine groups (CH) and the molecule consists of alternating single and double carbon bonds, this conjugated system of alternating double and single bonds allows them it to absorb light; particularly in the visible part of the electromagnetic spectrum.
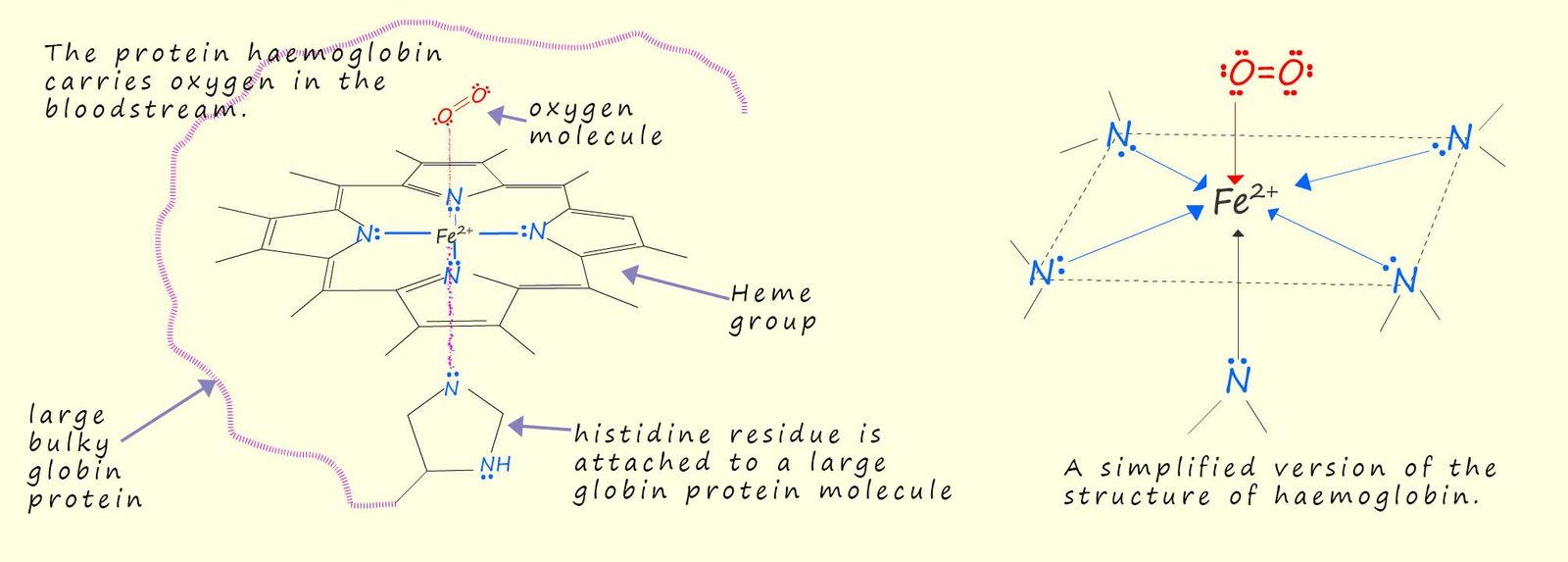
 While the ring structure of porphine is the basic starter unit found in many biological molecules, porphyrins are derivatives of porphine, these porphyrin molecules have the same basic conjugated ring structure as found in porphine but with added side groups or metal ions such as iron or magnesium added. Haemoglobin for example is the protein that is responsible for transporting oxygen in the bloodstream to ensure the body cells are able to respire. Haemoglobin consists of a heme molecule (see the image below). This heme molecule consists of a porphyrin ring system with an iron ion (Fe2+) in the centre of the molecule. The porphyrin ring system in the heme molecule acts as a tetradentate ligand, the heme molecule uses the four nitrogen atoms present in the porphyrin ring system to form four coordinate bonds to an iron ion (Fe2+). The iron ion (Fe2+) also forms a temporary and reversible coordinate bond with an oxygen molecule, which acts as a monodentate ligand. The Fe2+ ion has an octahedral arrangement around it with the heme molecule around its equator, with an oxygen molecule and a large protein molecule called globin which is bonded to a histidine residue (histidine is an amino acid), this histidine residue makes up the sixth and oxygen molecule occupy the remaining two sites in the octahedral structure, this is outlined in the diagram below:
While the ring structure of porphine is the basic starter unit found in many biological molecules, porphyrins are derivatives of porphine, these porphyrin molecules have the same basic conjugated ring structure as found in porphine but with added side groups or metal ions such as iron or magnesium added. Haemoglobin for example is the protein that is responsible for transporting oxygen in the bloodstream to ensure the body cells are able to respire. Haemoglobin consists of a heme molecule (see the image below). This heme molecule consists of a porphyrin ring system with an iron ion (Fe2+) in the centre of the molecule. The porphyrin ring system in the heme molecule acts as a tetradentate ligand, the heme molecule uses the four nitrogen atoms present in the porphyrin ring system to form four coordinate bonds to an iron ion (Fe2+). The iron ion (Fe2+) also forms a temporary and reversible coordinate bond with an oxygen molecule, which acts as a monodentate ligand. The Fe2+ ion has an octahedral arrangement around it with the heme molecule around its equator, with an oxygen molecule and a large protein molecule called globin which is bonded to a histidine residue (histidine is an amino acid), this histidine residue makes up the sixth and oxygen molecule occupy the remaining two sites in the octahedral structure, this is outlined in the diagram below:
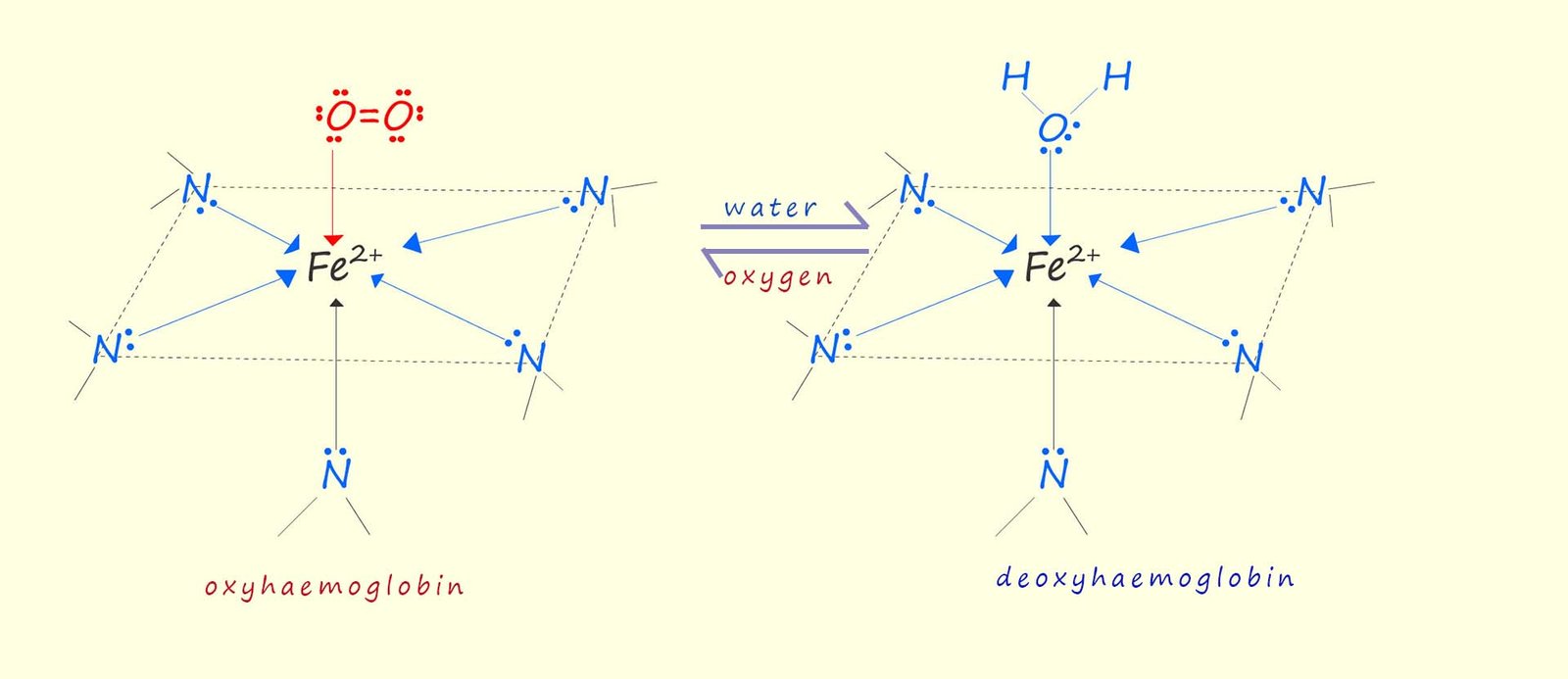
When an oxygen molecule is bonded to the iron ion (Fe2+) in the heme molecule the whole complex is called oxyhaemoglobin, it is this oxyhaemoglobin that gives blood its characteristic red colour. However once the oxygen is removed it is replaced by a water molecule, which like the oxygen molecule acts as a monodentate ligand, this whole complex is now called deoxyhaemoglobin and its colour changes to dark red. The structures of oxyhaemoglobin and deoxyhaemoglobin are shown below:
 Carbon monoxide (CO) gas is often described as the silent killer, it is an odourless, colourless gas which is produced when fuels like gas, oil, coal, and wood undergo incomplete combustion in boilers and stoves, often due to faulty or poorly serviced and ventilated appliances. It's a lethal deadly gas because it bonds strongly to the haemoglobin in the blood more readily than oxygen, effectively starving the body's cells of oxygen.
Carbon monoxide (CO) gas is often described as the silent killer, it is an odourless, colourless gas which is produced when fuels like gas, oil, coal, and wood undergo incomplete combustion in boilers and stoves, often due to faulty or poorly serviced and ventilated appliances. It's a lethal deadly gas because it bonds strongly to the haemoglobin in the blood more readily than oxygen, effectively starving the body's cells of oxygen.
In the UK carbon monoxide poisoning causes approximately 60 deaths annually and hospitalises around 4,000 people. Symptoms of CO poisoning can be easily mistaken for other illnesses, which makes it even more dangerous. Early signs include headaches, dizziness, nausea, shortness of breath, and fatigue. As exposure increases, symptoms may progress to confusion, chest pain, loss of consciousness, and ultimately death. Prolonged exposure even at low levels can lead to neurological problems and heart damage. To prevent CO poisoning it is important that all heating appliance are regularly maintained and that carbon monoxide detectors are installed in rooms containing log burners, gas boilers and fires.
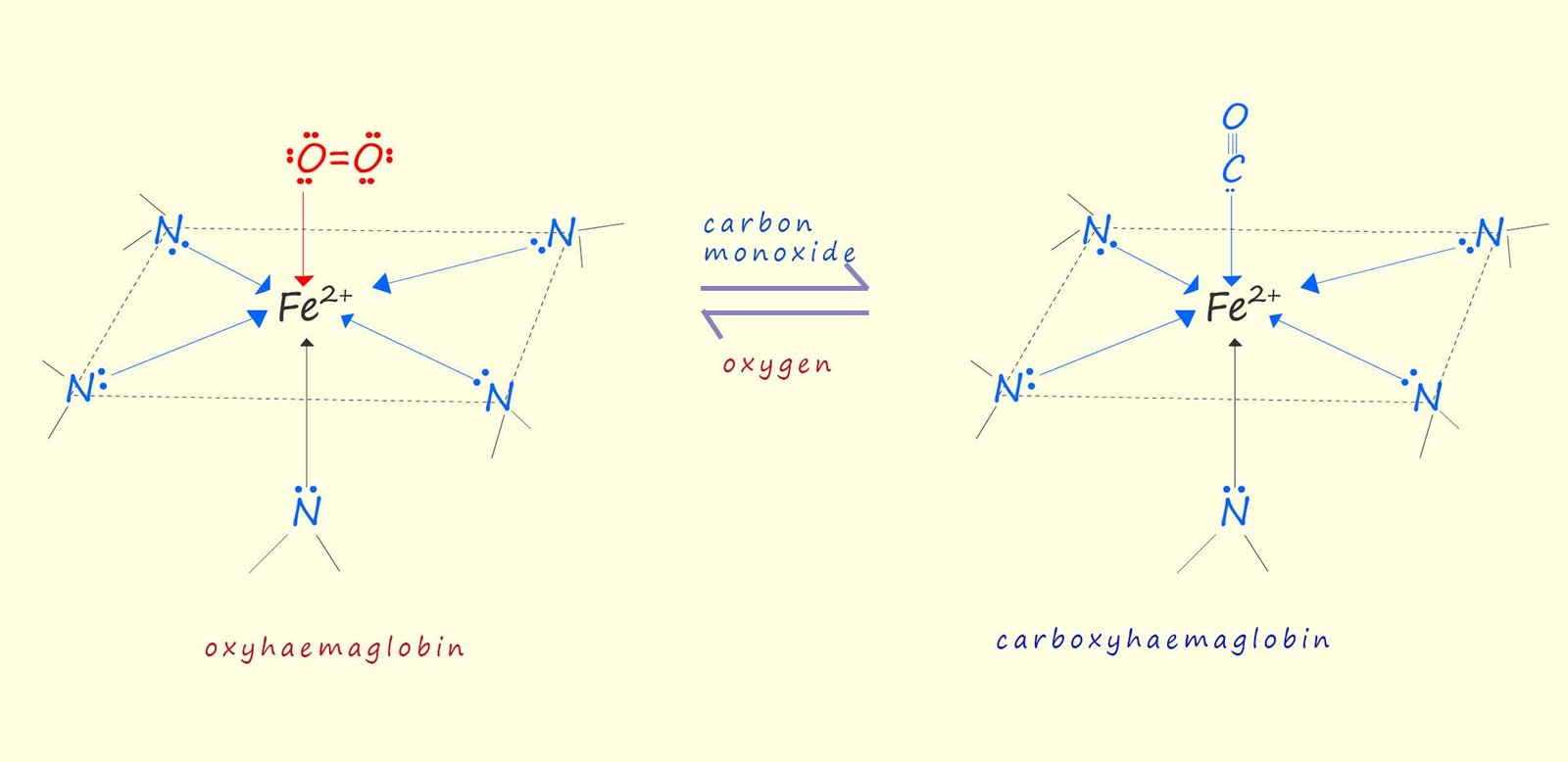
The carbon monoxide molecule has a lone pair of electrons that it can use to form a coordinate bond with the Fe2+ ion in the haemoglobin protein molecule, this is outlined in the image below, while the addition of carbon monoxide (CO) to haemoglobin to form carboxyhaemoglobin is shown as a reversible reaction in the image the reaction is by no means easy to reverse, the reason for this is simple, the CO-Fe bond is much stronger than the bond between the oxygen molecule and the Fe2+ ion, this stronger affinity for CO makes it very difficult for CO to dissociate from haemoglobin, even in the presence of oxygen. This effectively means when the CO bonds to haemoglobin it effectively blocks oxygen transport, leading to oxygen deprivation in the body tissues and if not quickly treated and reversed then death may follow.
Chlorophyll absorbs the light energy needed by plants to photosynthesise, chlorophyll contains at its core a porphyrin ring structure where the nitrogen atoms form four coordinate bonds to a magnesium ion (Mg2+), this is outlined below where a part of the chlorophyll structure is shown:
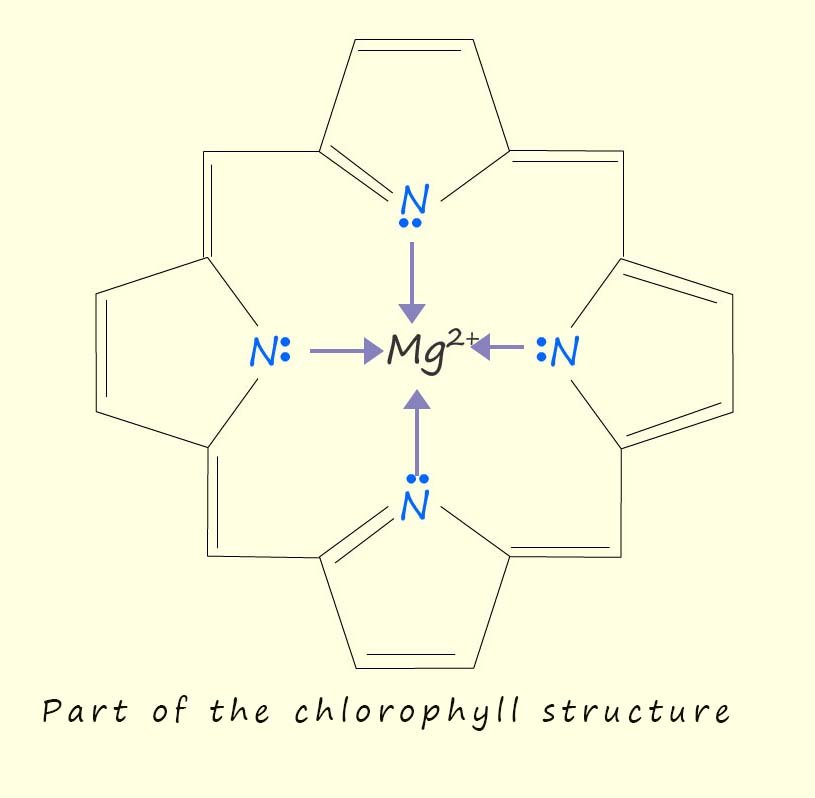 The presence of the conjugated ring system in the porphyrin ring structure is responsible for the absorption of the light needed for photosynthesis to take place.
The presence of the conjugated ring system in the porphyrin ring structure is responsible for the absorption of the light needed for photosynthesis to take place.