Stereoisomers are compounds which have the same structural formula but the atoms are arranged differently in 3d space. You may recall from the work you have already done in organic chemistry that there are two types of stereoisomers, these are:
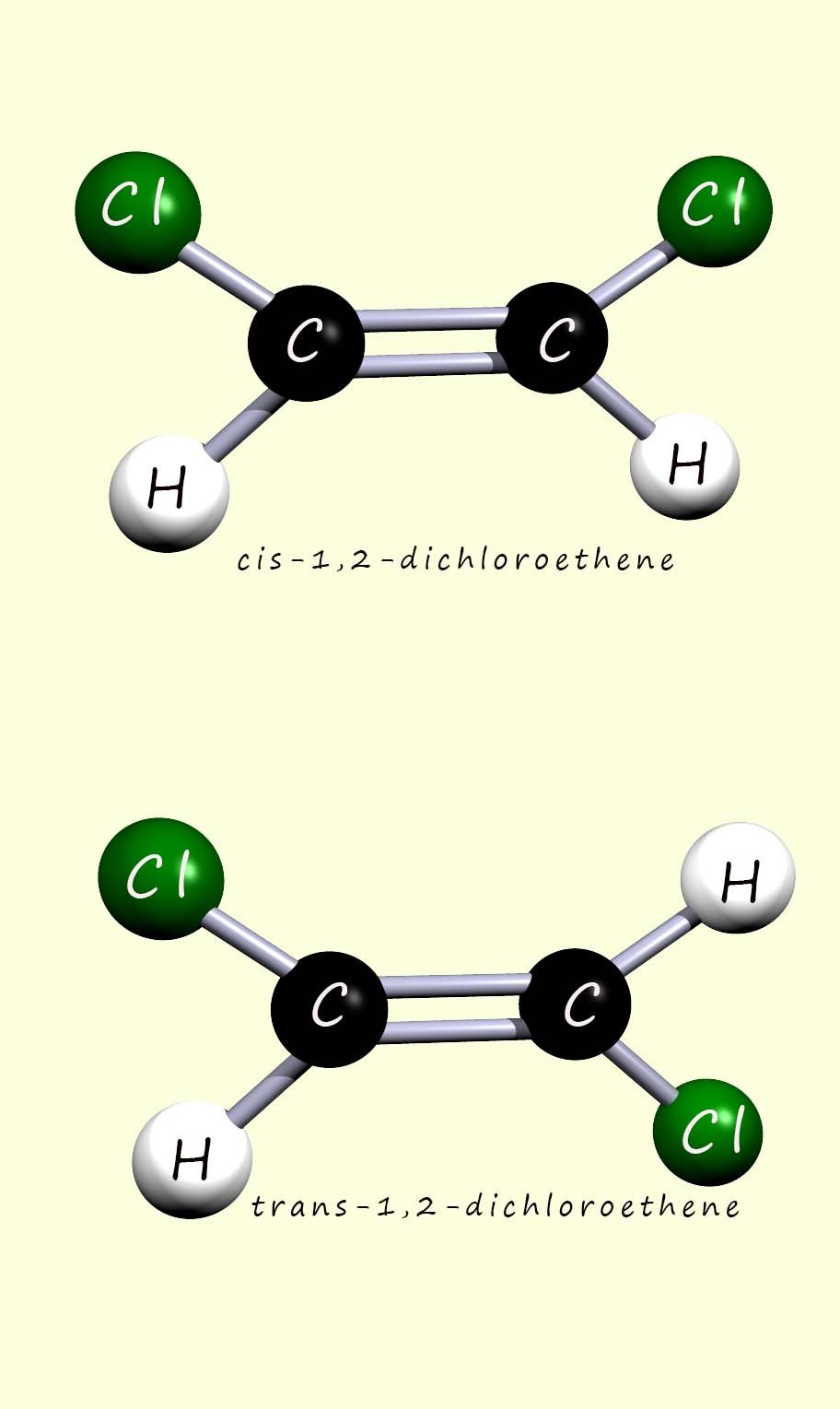 Now geometric isomerism is a form of stereoisomerism in which molecules have the same molecular formula but differ in how bonded groups or ligands are arranged in 3d space, for example around the carbon carbon double bond (C=C) in unsaturated molecules or around a metal atom or ion in a coordination complex e.g.
Now geometric isomerism is a form of stereoisomerism in which molecules have the same molecular formula but differ in how bonded groups or ligands are arranged in 3d space, for example around the carbon carbon double bond (C=C) in unsaturated molecules or around a metal atom or ion in a coordination complex e.g.
Coordination complexes can also exhibit geometric isomerism depending on how the attached ligands are arranged around the central metal ion e.g.
The image below shows two square planar complexes containing the metal platinum bonded to two ammonia ligands and to two chloride ligands, this complex is commonly called platin. Now from the image below you can clearly see that both of these complexes are flat or planar in shape and contain the four ligands arranged in a different way or order around the central platinum ion (Pt2+).
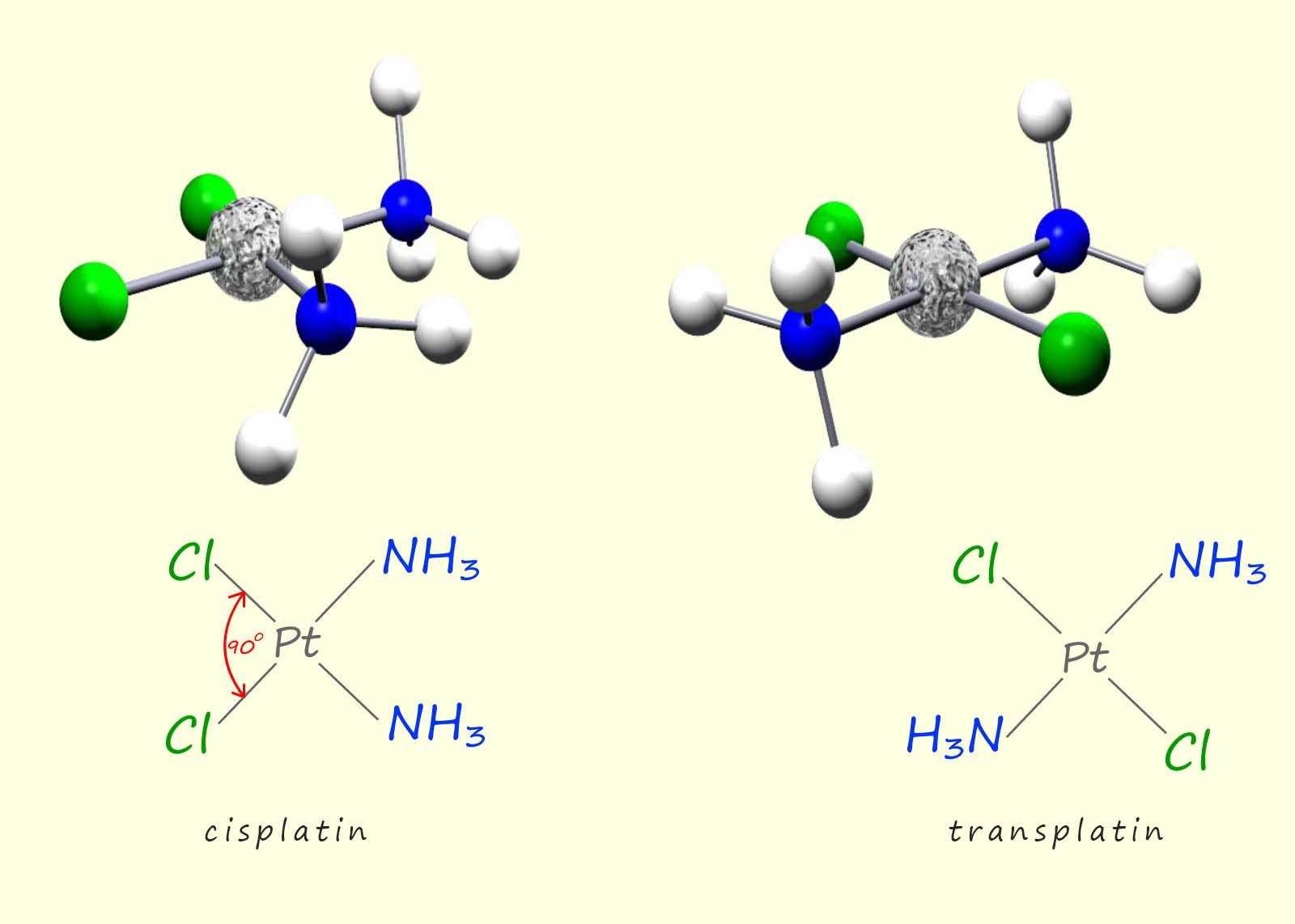
In the cis-isomer the two ammonia ligands or indeed the two chloride ligands are located on the same side of the molecule at an angle of 900 to each other while in the trans isomer the two ammonia ligands and also the two chloride ligands are located opposite each other and at an angle of 1800 to each other.
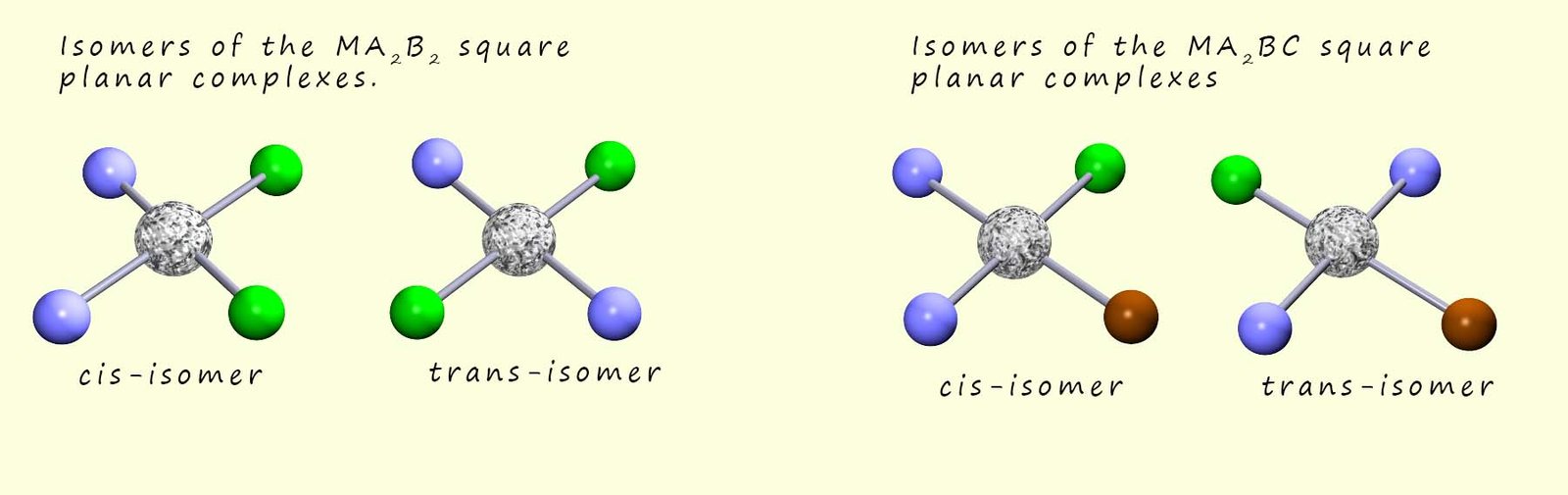
In general square planar complexes of the type MA2B2 and MA2BC; where M is a metal ion and A, B and C are ligands can show geometric isomerism, this is outlined in the image below:
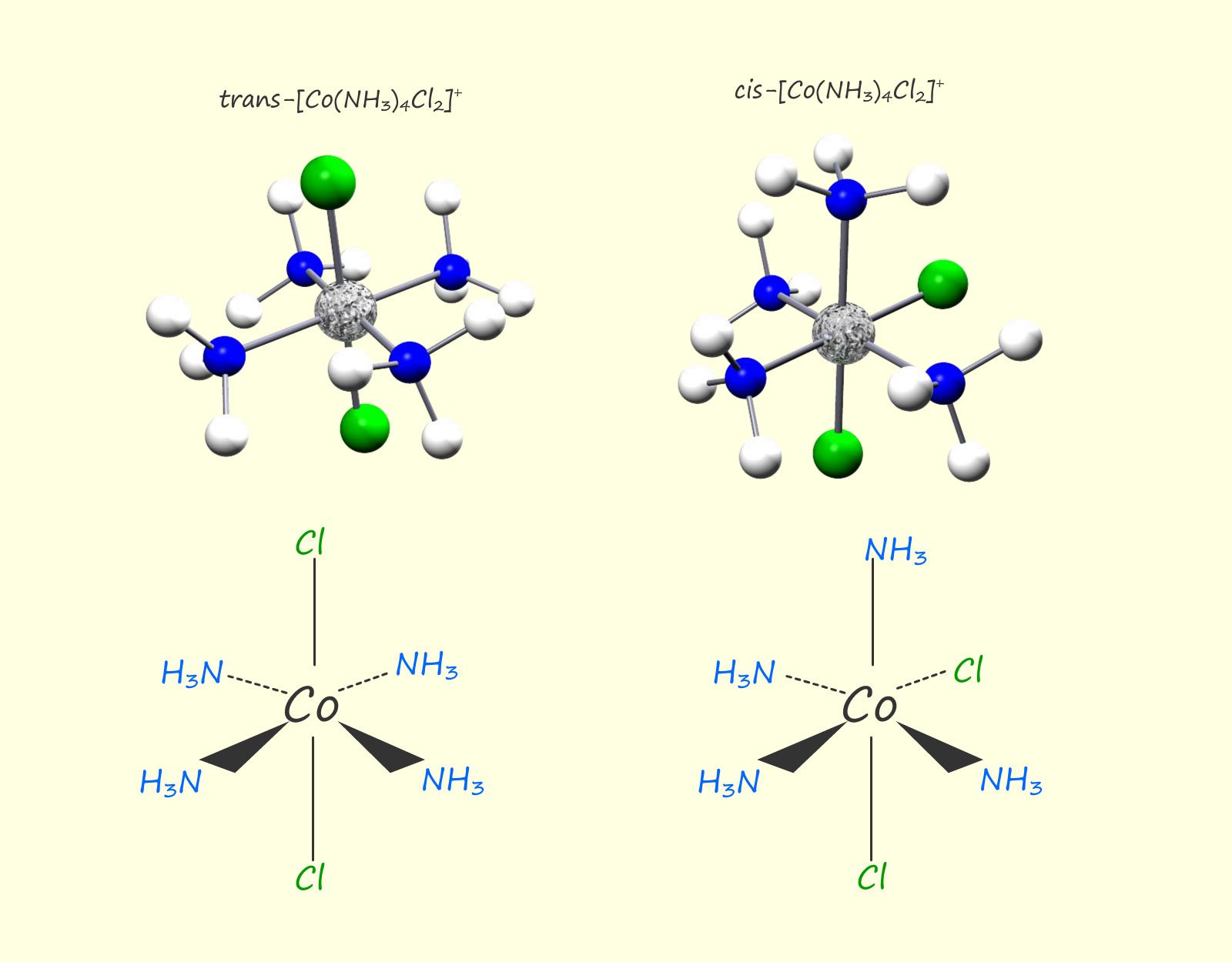
Octahedral complexes of the type MA4B2, where M is a metal ion and A and B are ligands will also exhibit geometric isomerism; for example the image below shows the cis and trans isomers for the complex ion [Co(NH3)4Cl2]+. In this complex there are four ammonia ligands and two chloride ligands bonded to a central cobalt ion (Co3+). In the cis geometric isomer the two chloride ions are beside or adjacent to each other, that is at an angle of 900 to each other and are on the same side of the molecule, while in the trans isomer the two chloride ligands are directly opposite each other at an angle of 180 0, this is outlined in the image below:
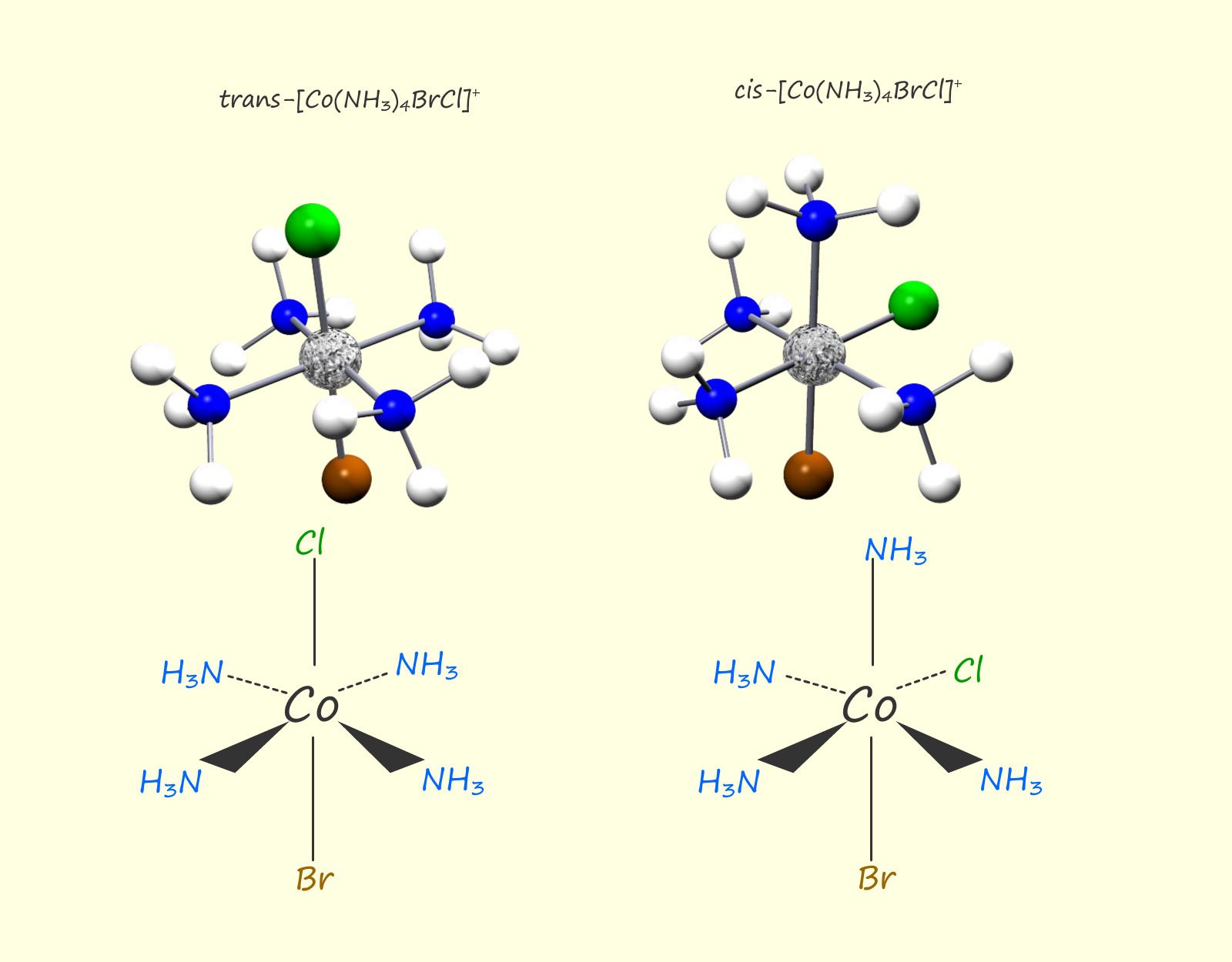
Cis and trans isomers are also possible for octahedral complexes of the type MA4BC, where A, B and C are all ligands, for example the image below shows structure of the cis and trans isomers for the complex [Co(NH3BrCl)]+:
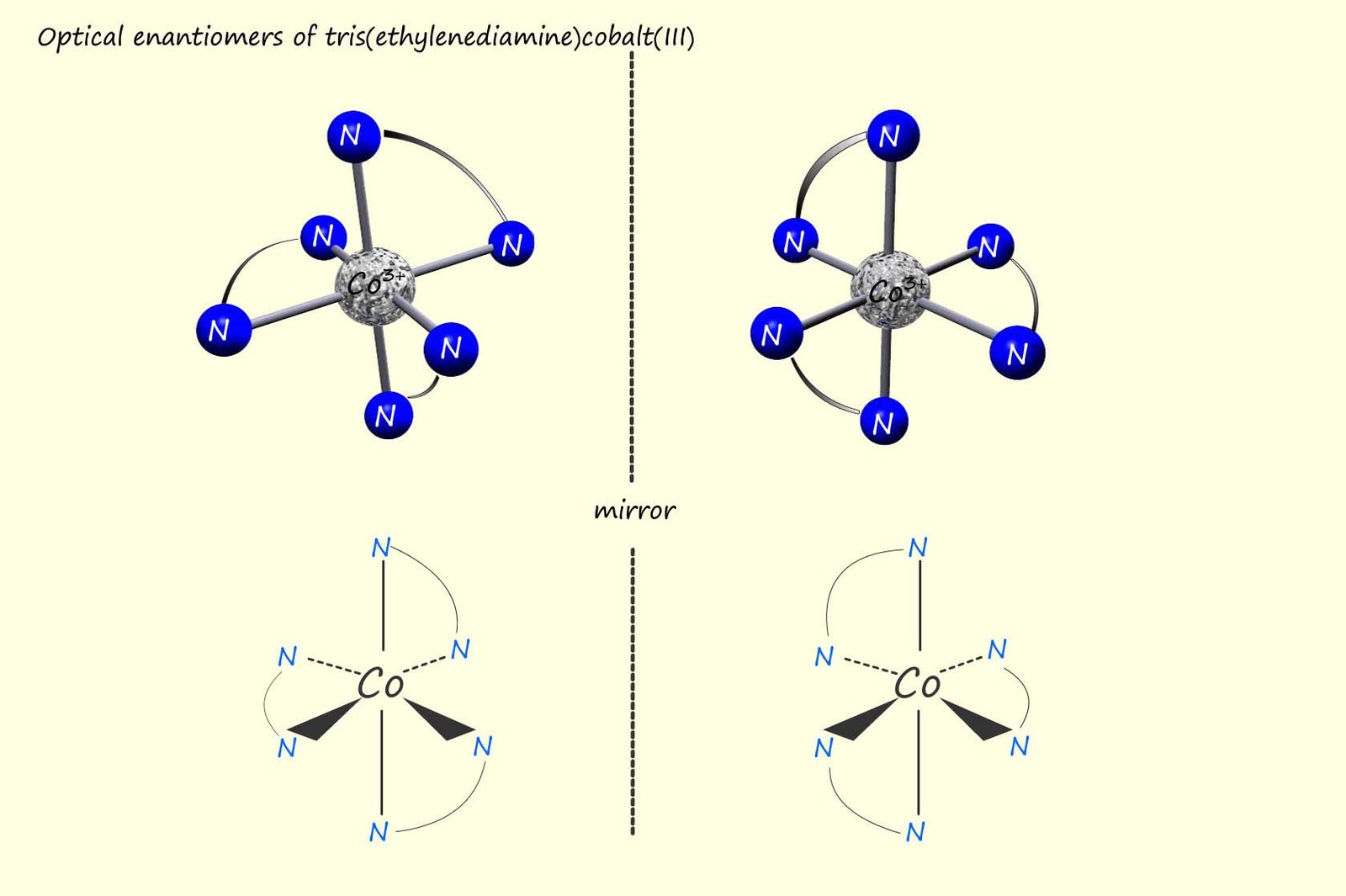
So far we have seen that transition metal ions can form octahedral and square planar complexes with a variety of small ligands such as water, ammonia and some of the halide ions, some of these complexes also existed as a pair of cis/trans geometrical isomers. However some octahedral complexes are also able to form optical active compounds, these optically active octahedral complexes contain bidentate ligands such as ethylenediamine (en).
The structure of the bidentate ligand ethylenediamine is shown below; now recall that a bidentate ligand is one that forms two coordinate bonds to a metal atom or ion.
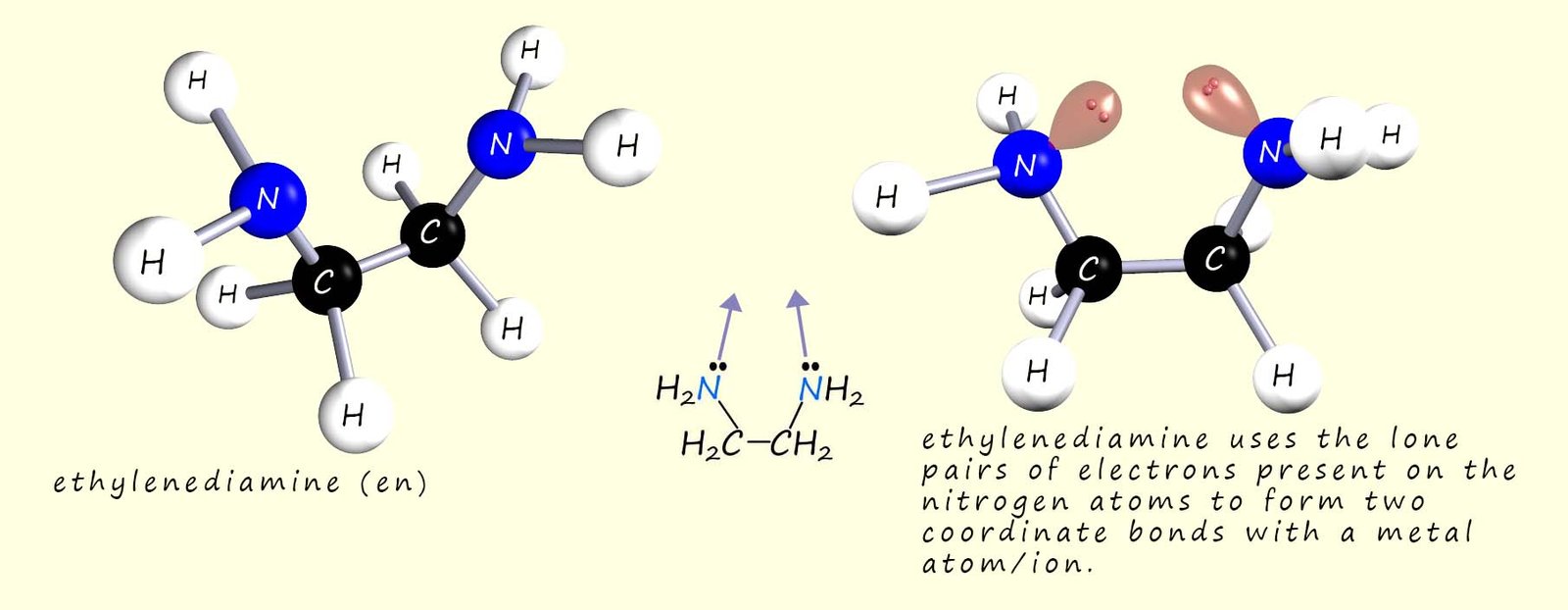
Ethylenediamine is a small molecule which contains two nitrogen atoms, these nitrogen atoms can use their lone pairs of electrons to form two coordinate bonds to a metal atom or ion, hence they are called bidentate ligands. An example of a complex containing this bidentate ligand is the octahedral tris(ethylenediamine)cobalt(III) -[Co(en)3]3+ complex; which contains three ethylenediamine ligands bonded to the central cobalt ion (Co3+), an image of this complex is shown below.
To try and help you visualise how the nitrogen atoms in the ethylenediamine ligand bond to the central cobalt ion I have shaded each of the nitrogen atoms in the three separate ethylenediamine ligands a slightly different colour.
![3d model and shorthand model of the [Co(en)3]3+ complex](images/ethylenediamine complex.jpg)
However it can be difficult to visualise the structure of this complex from the 3d model so a simplified version of the octahedral shaped complex is also shown. It is clear from this simplified structure that each of the ethylenediamine ligands is forming two coordinate bonds to the central cobalt ion. Now this complex can exist as a pair of enantiomers, that is a pair of optically active compounds or compounds that will rotate plane polarised light. The two enantiomers or mirror image forms of the tris(ethylenediamine)cobalt(III) complex are shown in the image below: