This page deals with the addition of a alkaline solutions such as sodium carbonate to solutions containing metal ions. To fully understand the ideas covered here it is important that you are very familiar with the acidity or hydrolysis reactions that take place in aqueous solutions containing metal ions with a 2+ and 3+ charge, click the link above to review this page if you need to. It is also worth quickly reviewing the page on precipitation reactions using sodium hydroxide and solutions of metal ions.
Solutions of metal carbonates such as sodium carbonate (Na2CO3) are basic and undergo similar reactions to the ones we have previously seen using alkaline solutions such as sodium hydroxide and ammonium hydroxide with hexaaqua metal complexes. The carbonate ion (CO32-) will react with solutions containing hexaaqua metal ions to form precipitates of the metal hydroxide in a similar way to other basic solutions such as sodium hydroxide and ammonia solution.
Now it is important to recall that the acidity of a hexaaqua transition metal complex depends on the oxidation state of the metal present in the complex. Now recall that hexaaqua metal complexes undergo an acidity reaction with water molecules. Equations to show the acidity reactions for hexaaqua metal(II) and metal(III) ions are shown below:
Recall that complexes which contain metal (II) ions typical have values for the acid dissociation constant Ka of between 10-6 and 10-11 while complexes containing metal (III) ions have a value of Ka in the range between 10-2 and 10-5. These very small values of Ka clearly indicate that the main species present in these two equilibrium reaction are the hexaaqua ions and that the position of equilibrium lies very much to the left hand side of the two equations above.
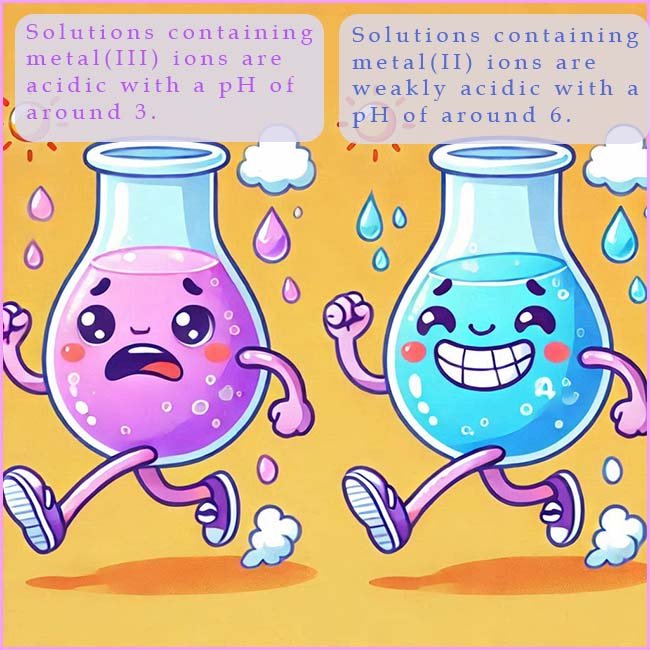
The acid dissociation contants (Ka) also shows that for complexes containing the metal (II) ions that around 1 in 10, 000 of the hexaaqua ions undergoes hydrolysis while for the metal (III) complexes about 1 in 1000 undergoes hydrolysis, or we can say that solutions of metal (II) ions will have a pH of around 6 while solutions of complexes containing metal (III) ions will have a pH of around 3. That is solutions containing a metal(III) ion are about 1000 times more acidic than solutions containing metal(II) ions.
Carbonate ions (CO32-) can react with hydronium ions (H3O+) or hydrogen ions (H+) in two separate reactions, in the first reaction shown below the carbonate ions (CO32-) react with hydrogen ions (H+) to form hydrogencarbonate ions (HCO3-) and water:
Now if the concentration of the hydrogen ions (H+) is sufficiently high then any hydrogencarbonate ions (HCO3-) present will undergo a further decomposition reaction to form carbon dioxide and water, an equation for this reaction is shown below:
Now as I am sure you are aware salts containing metals from group 1 of the periodic table, the alkali metals, are generally soluble in water. So metal carbonates such as sodium, potassium, and ammonium carbonate are all soluble and they will all dissolve to form weakly alkaline solutions. Most metal carbonates containing metal ions| with a 2+ or a 3+ charge are generally insoluble or only slightly soluble in water, although there can be exceptions to this general rule.
Solutions containing metal ions with a 2+ are not acidic enough, that is they do not produce a high enough concentration of hydrogen ions (H+) to push the both the equilibrium reactions above to the right hand side. So if a sodium carbonate solution is added to a solution containing a metal ions such as Cu2+, Fe2+, Co2+ or Mn2+ then an insoluble precipitate of the metal carbonate will be formed, but NO carbon dioxide gas will be produced since the concentration of hydrogen ions present is too low to decompose the hydrogencarbonate ions (HCO3-) into carbon dioxide gas, for example the image below shows the reaction that occurs when sodium carbonate solution is added to a copper(II) sulfate and an iron(II) sulfate solution, in each case a precipitate of the metal carbonate is produced but there are NO bubbles of carbon dioxide gas are released.
 IONS.jpg)
We can easily summarise these reactions as shown below using the precipitation reaction of copper sulfate solution with sodium carbonate solution as an example, note the sodium ions (Na+) and the sulfate ions (SO42-) are spectator ions and have been omitted from the equations below just to simplify them:
Or we can simplify this equation down to:
Aqueous solutions which contain metal ions with a 3+ charge are much more acidic than the same solutions which contain metal ions with a 2+ charge. So when a basic sodium carbonate solution is added to an aqueous solution containing a metal ion with a 3+ charge not only is a solid precipitate of the metal hydroxide formed but carbon dioxide gas is also released.
We can summarise the reactions of these aqueous solutions containing metals ions with a 3+ charge using the two examples in the image and equations below. The important points in this image and equations are that:
 IONS.jpg)